Page 110 - A Practical Introduction to Optical Mineralogy
P. 110
SILICATE MINERALS PYROXENE GROUP
Pumpellyite Sorosilicate EXTINCTION Oblique on cleavage with yAel and aAcl both = 4° to 30° seen on an (010)
2
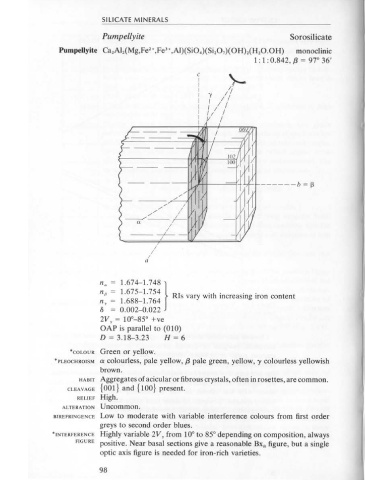
Pumpellyite Ca 2 Al 2 (Mg,Fe •,Fe'•,AI)(SiO.)(Si 2 0 7 )(0H) 2 (H 2 0.0H) monoclinic section.
TWINNING Sector twinning common on {001} and {100}.
1 : 1 : 0.842, f3 = 97° 36'
DISTINGUISHING Pumpellyite is similar to epidote but has a deeper colour and is biaxial
>- OCCURRENCE Pumpellyite is common in low grade regionally metamorphosed schists
c FEATURES positive (epidote is biaxial negative).
'I ;I and is characteristically developed in glaucophane-schist facies rocks.
I I I
I I I Pumpellyite may form by hydrothermal action in low temperature
1/ I veins, amygdales and altered mafic rocks.
v I
Pyroxene group Inosilicates
Orthorhombic pyroxenes (usually abbreviated to opx) and monoclinic
pyroxenes or clinopyroxenes (abbreviated to cpx) occur. A wide range.
of compositions is available. The general formula may be expressed as:
------b = 13
where X = Ca,Na, Y = Mg,Fe +,Ni,Li,Fe'+,Cr,Ti, and Z = Si,AI.
2
/
/ I
/ I In orthopyroxenes n is approximately 1 and virtually no trivalent or
/ I
---/---;-- monovalent ions are present; thus the formula reduces to Y 2 Z 2 0 6 or
{)( I
I (Mg,Fe,Mn),(Si,Al),0 6 . In clinopyroxenes n varies from 0 to 1, and the
--- -.L--
elemental substitutions must be such that the sum of the charges in X, Y
I and Z balances the six 0 2 - ions.
I
I Some pyroxenes have compositions that can be expressed in terms of
a
CaSiO,-FeSiO,-MgSiO, end members, and Figure 2.20 shows the main
monoclinic pyroxene minerals occurring in this field of composition. The
n. = 1.674-1.748 } principal change occurring in this group is a variation in symmetry
n p = 1.675-1.754 RI . h . . .
n y = 1. _1. s vary wtt mcreasmg tron content
688 764 Figure2.20 hedenbergite
8 = 0.002-0.022 Composition i
diagram for
2Vy = 10°-85° +ve
pyroxenes.
OAP is parallel to (010)
D = 3.18-3.23 H = 6
•coLOuR Green or yellow.
augite ferroaugite
*PLEOCHROISM a colourless, pale yellow, f3 pale green, yellow, y colourless yellowish
brown.
HABIT Aggregates of acicular or fibrous crystals, often in rosettes, are common.
CLEAVAGE {001} and {100} present.
RELIEF High.
ALTERATION Uncommon.
BIREFRINGENCE Low to moderate with variable interference colours from first order
greys to second order blues.
0 I!Kl
* INTERFERENCE Highly variable 2V y from 10° to 85° depending on composition, always
FIG URE MgSi0 3 FeSi0 3
positive. Near basal sections give a reasonable Bxa figure, but a single
clinoenstatite clinoferrosilite
optic axis figure is needed for iron-rich varieties. Mol per cent
98
99