Page 108 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 108
92 Chapter 7
Electrolytic Cells
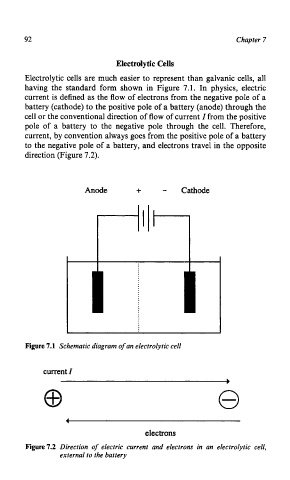
Electrolytic cells are much easier to represent than galvanic cells, all
having the standard form shown in Figure 7.1. In physics, electric
current is defined as the flow of electrons from the negative pole of a
battery (cathode) to the positive pole of a battery (anode) through the
cell or the conventional direction of flow of current I from the positive
pole of a battery to the negative pole through the cell. Therefore,
current, by convention always goes from the positive pole of a battery
to the negative pole of a battery, and electrons travel in the opposite
direction (Figure 7.2).
Anode + - Cathode
I I
Figure 7.1 Schematic diagram of an electrolytic cell
current I
7
electrons
Figure 7.2 Direction of electric current and electrons in an electrolytic cell,
external to the battery