Page 104 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 104
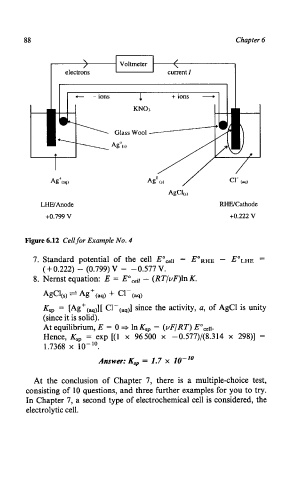
88 Chapter 6
\ /
r / Voltmeter
electrons current 1
r +ions -
c - ions 1
KNO3
Ag+(acl)
LHW Anode RHWCathode
+0.799 V +0.222 v
Figure 6.12 Cell for Example No. 4
-
7. Standard potential of the cell Eocell = E'RHE - E'LHE -
(+ 0.222) - (0.799) V = -0.577 V.
8. Nernst equation: E = EoceN - (RT/vF)ln K.
AgCl(s) + Ag + (as) + C1- (as)
Ksp = [Ag+(aq)][ Cl-(aq)] since the activity, a, of AgCl is unity
(since it is solid).
At equilibrium, E = 0 + In Ksp = (vF/RT) Eocell.
Hence, Ksp = exp [(l x 96500 x -0.577)/(8.314 x 298)] =
1.7368 x lo-''.
Answer: Ksp = 1.7 x lo-''
At the conclusion of Chapter 7, there is a multiple-choice test,
consisting of 10 questions, and three further examples for you to try.
In Chapter 7, a second type of electrochemical cell is considered, the
electrolytic cell.