Page 100 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 100
84 Chapter 6
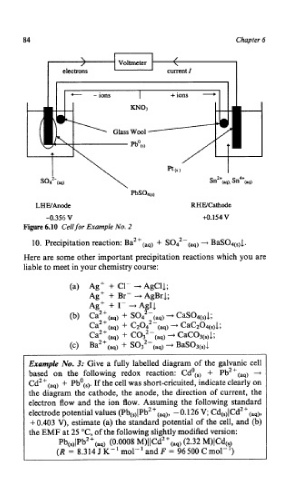
Voltmeter /
\
+ions -
current I
4- - ions
LHWAnodc RHWCathode
-0.356 V +0.154V
Figure 6.10 Cell for Example No. 2
10. Precipitation reaction: Ba2+(,,) + SO:-(aq) -, BaS04(,)J.
Here are some other important precipitation reactions which you are
liable to meet in your chemistry course:
~~ ~
Example No. 3: Give a fully labelled diagram of the galvanic cell
based on the following redox reaction: Cd',,) + Pb2+(,,) -,
Cd2+(,,) + Pb',,). If the cell was short-cricuited, indicate clearly on
the diagram the cathode, the anode, the direction of current, the
electron flow and the ion flow. Assuming the following standard
electrode potential values (Pb,,)lPb2+(,q), -0.126 V; Cd(,)ICd2+(,q),
+0.403 V), estimate (a) the standard potential of the cell, and (b)
the EMF at 25 "C, of the following slightly modified version:
Pb(,)l Pb2 + (aq) (0 -0008 M) I I Cd2 + (aq) (2.3 2 M) I Cd(y
(R = 8.314 J K-' mol-' and F = 96 500 C mol- )