Page 101 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 101
Electrochemistry I: Galvanic Cells 85
Solution:
1. There is no mention of 'electrolysis' or 'electrolysed'; therefore
the cell in question is a galvanic cell.
2. For part (a), since a balanced chemical equation is given, this
determines which electrode will act as the cathode, and which
electrode will act as the anode. Therefore, the oxidation state of
each species must be written down:
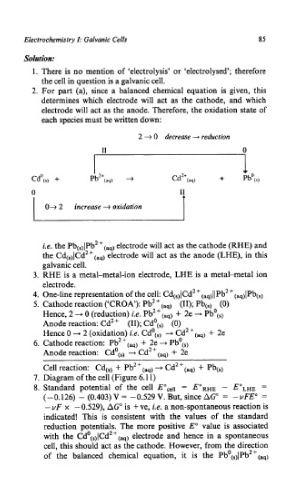
2 + 0 decrease -, reduction
0
CdO(,, +
0 I1
T
O+ 2 increase + oxidation
i.e. the Pb(,)lPb2+(,,) electrode will act as the cathode (RHE) and
the Cd(,,lCdZ+(,q) electrode will act as the anode (LHE), in this
galvanic cell.
3. RHE is a metal-metal-ion electrode, LHE is a metal-metal ion
electrode.
4. One-line representation of the cell: Cd(,)(Cd2+(aq)IIPbZf(aq)IPb(,)
5. Cathode reaction ('CROA'): Pb2+(aq) (11); Pb(,) (0)
Hence, 2 -+ 0 (reduction) i.e. Pb2+(aq) + 2e -+ Pbo(,)
Anode reaction: Cd2+ (11); Cdo(,) (0)
Hence 0 -+ 2 (oxidation) i.e. Cdo(,) -+ Cd2+(,,) + 2e
6. Cathode reaction: Pb2+(aq) + 2e -+ Pb',,)
Anode reaction: Cd',,) --+ Cd2+(aq) + 2e
Cell reaction: Cd(,) + Pb2+(aq) -+ Cd2+(aq) + Pb,,)
7. Diagram of the cell (Figure 6.1 1)
-
8. Standard potential of the cell Eocell = EoRHE - EoLHE -
(-0.126) - (0.403) V = -0.529 V. But, since AGO = -vFEo =
- uF x - 0.529), AGO is + ve, i.e. a non-spontaneous reaction is
indicated! This is consistent with the values of the standard
reduction potentials. The more positive Eo value is associated
with the Cdo(&d2+ electrode and hence in a spontaneous
cell, this should act as the cathode. However, from the direction
of the balanced chemical equation, it is the Pb0(,)lPb2+(aq)