Page 109 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 109
Electrochemistry 11: Electrolytic Cells 93
A useful way to remember this is the mnemonic ‘CNAP’, i.e. cathode-
negative anode-positive. In electrolytic cells, the electrode connected
to the negative pole of the battery is the cathode and the electrode
connected to the positive pole of the battery is the anode.
Summary:
(a) CROA: applicable to both galvanic and electrolytic cells.
(b) CNAP: applicable to electrolytic cells only.
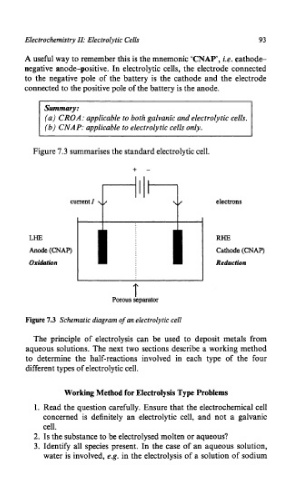
Figure 7.3 summarises the standard electrolytic cell.
+ -
Ill
electrons
LHE RHE
Anode (CNAP) Cathode (CNAP)
Oxidation Reduction
T
Porous separator
Figure 7.3 Schematic diagram of an electrolytic cell
The principle of electrolysis can be used to deposit metals from
aqueous solutions. The next two sections describe a working method
to determine the half-reactions involved in each type of the four
different types of electrolytic cell.
Working Method for Electrolysis Type Problems
1. Read the question carefully. Ensure that the electrochemical cell
concerned is definitely an electrolytic cell, and not a galvanic
cell.
2. Is the substance to be electrolysed molten or aqueous?
3. Identify all species present. In the case of an aqueous solution,
water is involved, e.g. in the electrolysis of a solution of sodium