Page 114 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 114
98 Chapter 7
+
7
electrons
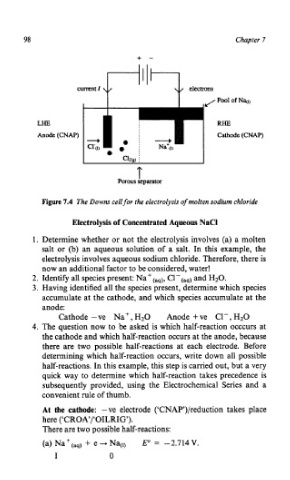
LHE RHE
Anode (CNAP) Cathode (CNAP)
T
Porous separator
Figure 7.4 The Downs cell for the electrolysis of molten sodium chloride
Electrolysis of Concentrated Aqueous NaCl
1. Determine whether or not the electrolysis involves (a) a molten
salt or (b) an aqueous solution of a salt. In this example, the
electrolysis involves aqueous sodium chloride. Therefore, there is
now an additional factor to be considered, water!
2. Identify all species present: Na+(aq), Cl-(aql and H20.
3. Having identified all the species present, determine which species
accumulate at the cathode, and which species accumulate at the
anode:
Cathode -ve Na+, H20 Anode +ve C1-, H20
4. The question now to be asked is which half-reaction occcurs at
the cathode and which half-reaction occurs at the anode, because
there are two possible half-reactions at each electrode. Before
determining which half-reaction occurs, write down all possible
half-reactions. In this example, this step is carried out, but a very
quick way to determine which half-reaction takes precedence is
subsequently provided, using the Electrochemical Series and a
convenient rule of thumb.
At the cathode: - ve electrode (‘CNAP’)/reduction takes place
here (‘CROA’/‘OILRIG’).
There are two possible half-reactions:
(a) Na+(,,) + e + Na(l1 E“ = -2.714V.
I 0