Page 119 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 119
Electrochemistry II: Electrolytic Cells 103
i.e. generating H+ ions at the cathode. The leads to only one
posssible reaction at the cathode: 2H+(aq) + 2e + Hz(~).
At the anode, there are two possible half-reactions:
(a) 2SOz-(aq) + S20:-(aq) + 2e E" = -2.05 V.
SV1 Peroxydisulfate anion
This is a very unfavourable half-reaction (as shown by a large
-ve E" value, i.e. AGO very +ve, implying a non-sponta-
neous reaction). Sulfur is not oxidised, as in the VI oxidation
state, it has the stable inert gas core configuration of me], i.e.
oxidation of the sulfate will not occur, and water will instead
be oxidised:
(b) 2H,O(aq) 4 Oqg) + 4H+(aq) + 4e E" = - 1.23 V.
1-11 0 I
t
I
-2 to 0 (oxidation)
Cathode reaction: 2H+(,q) + 2e -+ H2ig)
Anode reaction: 2H20(aq)+02(g) + 4H (aq) + 4e
Multiply by two: Cathode reaction: 4H+(aq) + 4e + 2H2(g)
Anode reaction: 2H20(aq)+O*(g) + 4H+(aq) + 4e
Cell reaction: 2H20(aq) -+ 2H2(g) + 02(g)
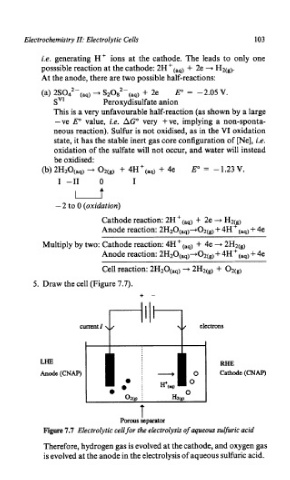
5. Draw the cell (Figure 7.7).
+-
I
I I
LHE RHE
Anode (CNAP) Cathode (CNAP)
T
Porous separator
Figure 1.7 Electrolytic cell for the electrolysis of aqueous sulfuric acid
Therefore, hydrogen gas is evolved at the cathode, and oxygen gas
is evolved at the anode in the electrolysis of aqueous sulfuric acid.