Page 123 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 123
Electrochemistry II: Electrolytic Cells 107
Therefore water is oxidised at the anode, evolving oxygen gas,
according to the half-reaction: 2H,O(aq) -+ 02(g) + 4Hf(aq) + 4e.
6. Write down the two half-reactions:
Cathode reaction: CU2+(aq) + 2e -+ CU'(~)
Anode reaction: 2H20(,q)+02(g) + 4H + (aq) + 4e
Multiply by two: Cathode reaction: 2Cu2+(aq) + 4e -+ 2Cuo(,)
Anode reaction: 2H20(aq)+02(g) + 4H + (aq) + 4e
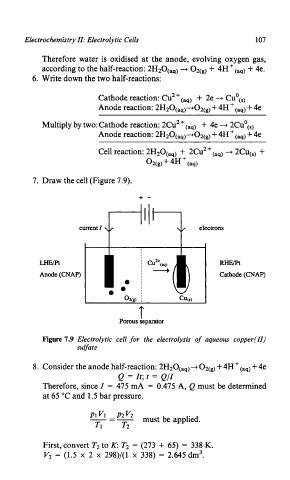
7. Draw the cell (Figure 7.9).
+-
T Y
current I
LHE/pt RHE/Pt
Anode (CNAP) Cathode (CNAP)
T
Porous separator
Figure 7.9 Electrolytic cell for the electrolysis of aqueous copper(II)
suvate
8. Consider the anode half-reaction: 2HzO(aq) -, 02(g) + 4H + (as) + 4e
Q = It; t = Q/I
Therefore, since I = 475 mA = 0.475 A, Q must be determined
at 65 "C and 1.5 bar pressure.
'!?!!!. -'* must be applied.
-
TI T2
First, convert T2 to K: T2 = (273 + 65) = 338 K.
V2 = (1.5 x 2 x 298)/(1 x 338) = 2.645 dm3.