Page 118 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 118
102 Chapter 7
be evolved at the anode. In this example, let us assume that the
acid is very concentrated:
Cathode reaction: 2H+(,q) + 2e- + H2(g)
Anode reaction: 2Cl-(aq> + C12(g) + 2e
Cell reaction: 2H+(,q) + 2Cl-(aq) -+ C1,(,) + Hqg)
The electrolysis of hydrochloric acid is very important in stres-
sing the role of the initial acid ionisation reaction and the effect
of concentration in these types of electrolytic cells.
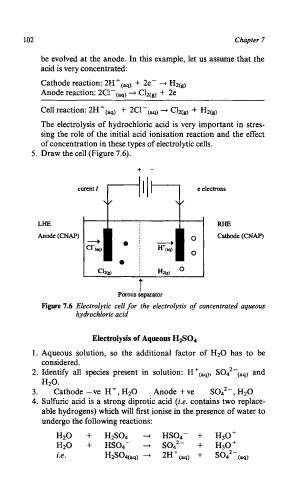
5. Draw the cell (Figure 7.6).
+ -
LHE RHE
Anode (CNAP) Cathode(CNAP)
Cl2Cs) i Hz(g) O
T
Porous separator
Figure 7.6 Electrolytic cell for the electrolysis of concentrated aqueous
hydrochloric acid
Electrolysis of Aqueous H2S04
1. Aqueous solution, so the additional factor of H20 has to be
considered.
2. Identify all species present in solution: H+(,,, SOz-(aq) and
H20.
3. Cathode -ve Hf, H20 Anode +ve SOZ-, H20
4. Sulfuric acid is a strong diprotic acid (Le. contains two replace-
able hydrogens) which will first ionise in the presence of water to
undergo the following reactions:
H20 + H2S04 --+ HS04- + H30+
H20 + HS04- -+ so42- + H30+
i.e. H2S04(aq) 2H+(aq) + so42-(aq)