Page 116 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 116
100 Chapter 7
+
LHE
Anode (CNAP) Cathode (CNAP)
T
Porous separator
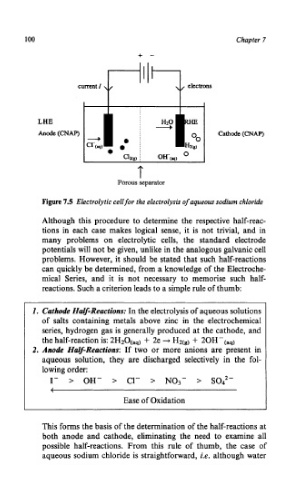
Figure 7.5 Electrolytic cell for the electrolysis of aqueous sodium chloride
Although this procedure to determine the respective half-reac-
tions in each case makes logical sense, it is not trivial, and in
many problems on electrolytic cells, the standard electrode
potentials will not be given, unlike in the analogous galvanic cell
problems. However, it should be stated that such half-reactions
can quickly be determined, from a knowledge of the Electroche-
mical Series, and it is not necessary to memorise such half-
reactions. Such a criterion leads to a simple rule of thumb:
1. Cathode Half-Reactions: In the electrolysis of aqueous solutions
of salts containing metals above zinc in the electrochemical
series, hydrogen gas is generally produced at the cathode, and
the half-reaction is: 2H20(,q) + 2e -+ H2(g) + 20H-(,)
2. Anode Hal’Reactions: If two or more anions are present in
aqueous solution, they are discharged selectively in the fol-
lowing order:
I- > OH- > C1- > NO3- > S042-
\
Ease of Oxidation
This forms the basis of the determination of the half-reactions at
both anode and cathode, eliminating the need to examine all
possible half-reactions. From this rule of thumb, the case of
aqueous sodium chloride is straightforward, i.e. although water