Page 121 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 121
Electrochemistry 11: Electrolytic Cells 105
+-
T
Porous Separator
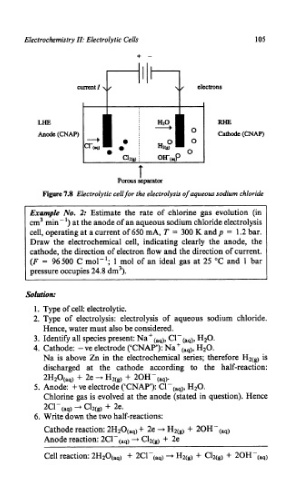
Figure 7.8 Electrolytic cell for the electrolysis of aqueous sodium chloride
~
Example No. 2: Estimate the rate of chlorine gas evolution (in
cm3 min-’) at the anode of an aqueous sodium chloride electrolysis
cell, operating at a current of 650 mA, T = 300 K andp = 1.2 bar.
Draw the electrochemical cell, indicating clearly the anode, the
cathode, the direction of electron flow and the direction of current.
(F = 96500 C mol-’; 1 mol of an ideal gas at 25 “C and 1 bar
pressure occupies 24.8 dm3).
Solution:
1. Type of cell: electrolytic.
2. Type of electrolysis: electrolysis of aqueous sodium chloride.
Hence, water must also be considered.
3. Identify all species present: Na+(,q), C1-(aq), H20.
4. Cathode: -ve electrode (‘CNAP’): Naf(aq), H20.
Na is above Zn in the electrochemical series; therefore H2(!) is
discharged at the cathode according to the half-reaction:
2H20(aq) + 2e --+ H2(g) + 20H-(aq).
5. Anode: + ve electrode (‘CNAP’): Cl-(aq), H20.
Chlorine gas is evolved at the anode (stated in question). Hence
2Cl-(aql -+ C12(g) + 2e.
6. Write down the two half-reactions:
Cathode reaction: 2H,O(aq) + 2e -+ H2(g) + 20H - (as)
Anode reaction: 2Cl-(aq) -, C12(,) + 2e
Cell reaction: 2H20(aq) + 2Cl-(aql 4 H2(g) + Clz(g) + 20H-(aq)