Page 125 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 125
Electrochemistry 11: Electrolytic Cells 109
Cathode reaction: Au3 + (as) + 3e 4 Au'(,)
Anode reaction: 2H20(aq>+02(g) + 4H +(as) + 4e
Multiply by four: Cathode reaction: 4Au3 +(as) + 12e 4 4Auo(,)
Multiply by three: Anode reaction: 6H20(aq)-+302(g)+l 2W(aq)+l2e
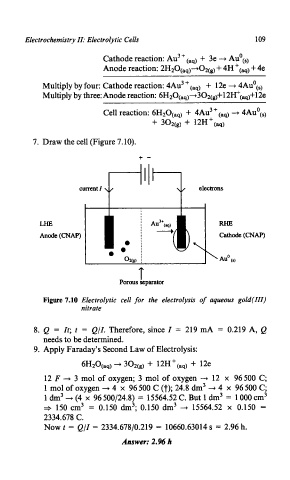
7. Draw the cell (Figure 7.10).
+-
LIE
Anode (CNAP) Cathode (CNAP)
T
Porous separator
Figure 7.10 Electrolytic cell for the electrolysis of aqueous gold(III)
nitrate
8. Q = It; t = Q/Z. Therefore, since Z = 219 mA = 0.219 A, Q
needs to be determined.
9. Apply Faraday's Second Law of Electrolysis:
6H,O(aq) -+ 30qg) + 12H+(aq) + 12e
12 F -+ 3 mol of oxygen; 3 mol of oxygen -+ 12 x 96 500 C;
1 mol of oxygen -+ 4 x 96500 C (t); 24.8 dm3 + 4 x 96500 C;
1 dm3 -+ (4 x 96 500/24.8) = 15564.52 C. But 1 dm3 = 1 000 cm3
+ 150 cm3 = 0.150 dm3; 0.150 dm3 --+ 15564.52 x 0.150 =
2334.678 C.
Now t = Q/Z = 2334.678/0.219 = 10660.63014 s = 2.96 h.
Answer: 2.96 h