Page 133 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 133
Chemical Kinetics I 117
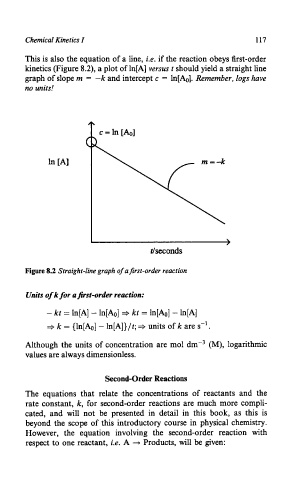
This is also the equation of a line, i.e. if the reaction obeys first-order
kinetics (Figure 8.2), a plot of ln[A] versus t should yield a straight line
graph of slope m = -k and intercept c = ln[Ao]. Remember, logs have
no units!
.
t/seconds
Figure 8.2 Straight-line graph of a first-order reaction
Units of k for a first-order reaction:
- kt = ln[A] - ln[Ao] j kt = In[&] - ln[A]
+ k = {In[&] - ln[A]}/t; j units of k are s-'.
Although the units of concentration are mol dm-3 (M), logarithmic
values are always dimensionless.
Second-Order Reactions
The equations that relate the concentrations of reactants and the
rate constant, k, for second-order reactions are much more compli-
cated, and will not be presented in detail in this book, as this is
beyond the scope of this introductory course in physical chemistry.
However, the equation involving the second-order reaction with
respect to one reactant, i.e. A -+ Products, will be given: