Page 132 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 132
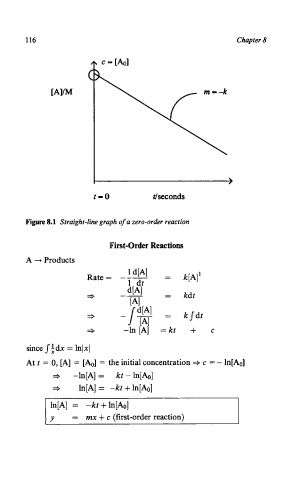
116 Chapter 8
Figure 8.1 Straight-line graph of a zero-order reaction
First-Order Reactions
A --+ Products
Rate = kPl'
kdt
- ksdt
-
= kt + C
since Jidx = lnlxl
At t = 0, [A] = [Ao] = the initial concentration + c = - ln[Ao]
j -ln[A] = kt-In[&]
* ln[A] = -kt + In[&]
ln[A] = -kt + In[&]
= rnx + c (first-order reaction)
I y