Page 139 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 139
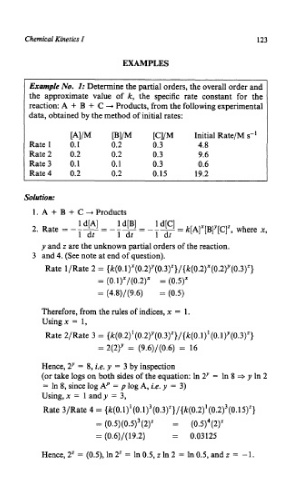
Chemical Kinetics I 123
EXAMPLES
Example No. I: Determine the partial orders, the overall order and
the approximate value of k, the specific rate constant for the
reaction: A + B + C -+ Products, from the following experimental
data, obtained by the method of initial rates:
[A]/M [B]/M [C]/M Initial Rate/M s-l
Rate 1 0.1 0.2 0.3 4.8
Rate2 0.2 0.2 0.3 9.6
Rate 3 0.1 0.1 0.3 0.6
Rate4 0.2 0.2 0.15 19.2
Solution:
1. A + B + C + Products
y and z are the unknown partial orders of the reaction.
3 and 4. (See note at end of question).
Rate 1/Rate 2 = {k(0.1)"(0.2)Y(0.3)z}/{k(0.2)x(0.2)Y(0.3)2}
= (0.1)x/(0.2)x = (0.5)x
= (4.8)/(9.6) = (0.5)
Therefore, from the rules of indices, x = 1.
Using x = 1,
Rate 2/Rate 3 = (k(0.2)' (0.2)Y(0.3)z}/{k(0.1)' (0.1)Y(0.3)2}
= 2(2)' = (9.6)/(0.6) = 16
Hence, 2y = 8, i.e. y = 3 by inspection
(or take logs on both sides of the equation: In 2Y = In 8 =$ y In 2
= In 8, since log Ap = p log A, i.e. y = 3)
Using, x = 1 and y = 3,
Rate 3/Rate 4 = {k(O.l)' (0.1)3(0.3)'}/{k(0.2)' (0.2)3(0.15)z}
= (0.5)(0.5)3(2)z = (0.5)4(2)"
= (0.6)/( 19.2) = 0.03125
Hence, 2" = (0.5), In 2" = In 0.5, z In 2 = In 0.5, and z = - 1.