Page 142 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 142
126 Chapter 8
CH3 CH3 H- CH2 CH3
Ratedetcrminina
-H
+
x" Br6-
1
Br- CH2 CH3 En01
CH2 CH3
A + H+
BCH2 CH3
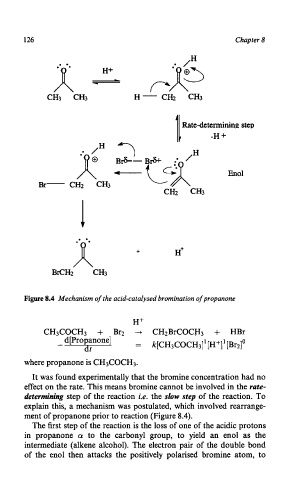
Figure 8.4 Mechanism of the acid-catalysed bromination of propanone
H+
CH3COCH3 + Br2 -+ CHzBrCOCH3 + HBr
- d[Propanone]
dt = k[CH3COCH3]'[H+]'[Brz]'
where propanone is CH3COCH3.
It was found experimentally that the bromine concentration had no
effect on the rate. This means bromine cannot be involved in the rate-
determining step of the reaction i.e. the slow step of the reaction. To
explain this, a mechanism was postulated, which involved rearrange-
ment of propanone prior to reaction (Figure 8.4).
The first step of the reaction is the loss of one of the acidic protons
in propanone (Y to the carbonyl group, to yield an enol as the
intermediate (alkene alcohol). The electron pair of the double bond
of the enol then attacks the positively polarised bromine atom, to