Page 147 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 147
Chemical Kinetics 11 131
The Steric Factor, p
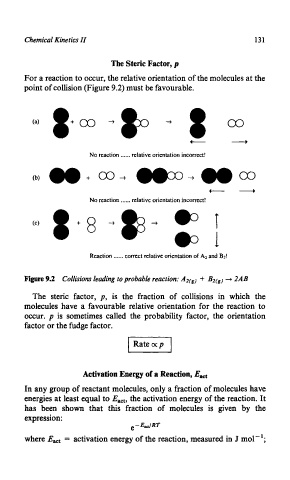
For a reaction to occur, the relative orientation of the molecules at the
point of collision (Figure 9.2) must be favourable.
No reaction ...... relative orientation incorrect!
-d
No reaction ...... relative orientation incorrect!
Reaction ...... correct relative orientation of A:! and B?!
Figure 9.2 Collisions leading to probable reaction: AZ(g) -+ BZ(g) + 2AB
The steric factor, p, is the fraction of collisions in which the
molecules have a favourable relative orientation for the reaction to
occur. p is sometimes called the probability factor, the orientation
factor or the fudge factor.
Activation Energy of a Reaction, EPCt
In any group of reactant molecules, only a fraction of molecules have
energies at least equal to Eact, the activation energy of the reaction. It
has been shown that this fraction of molecules is given by the
expression:
e- EdRT
where E,, = activation energy of the reaction, measured in J mol-';