Page 149 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 149
Chemical Kinetics 11 133
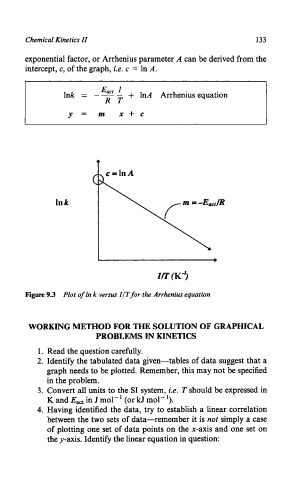
exponential factor, or Arrhenius parameter A can be derived from the
intercept, c, of the graph, i.e. c = In A.
Eat 1
Ink = -- - + 1nA Arrhenius equation
RT
Y = m x+c
ID' (K-3
Figure 9.3 Plot of In k versus ZIT for the Arrhenius equation
WORKING METHOD FOR THE SOLUTION OF GRAPHICAL
PROBLEMS IN KINETICS
1. Read the question carefully.
2. Identify the tabulated data given-tables of data suggest that a
graph needs to be plotted. Remember, this may not be specified
in the problem.
3. Convert all units to the SI system, i.e. T should be expressed in
K and Eact in J mol- ' (or kJ mol- ').
4. Having identified the data, try to establish a linear correlation
between the two sets of data-remember it is not simply a case
of plotting one set of data points on the x-axis and one set on
the y-axis. Identify the linear equation in question: