Page 146 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 146
130 Chapter 9
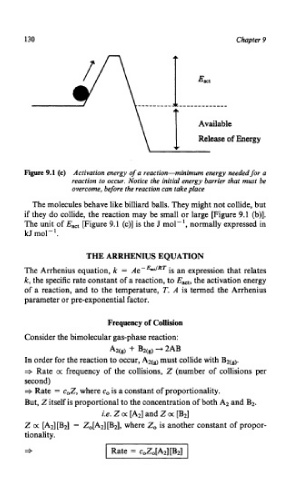
Figure 9.1 (c) Activation energy of a reaction-minimum energy needed for a
reaction to occur. Notice the initial energy barrier that must be
overcome, before the reaction can take place
The molecules behave like billiard balls. They might not collide, but
if they do collide, the reaction may be small or large [Figure 9.1 (b)].
The unit of Eact [Figure 9.1 (c)] is the J mol-', normally expressed in
kJ mol-'.
THE ARRHENIUS EQUATION
The Arrhenius equation, k = Ae-E"JRT is an expression that relates
k, the specific rate constant of a reaction, to ,!?act, the activation energy
of a reaction, and to the temperature, T. A is termed the Arrhenius
parameter or pre-exponential factor.
Frequency of Collision
Consider the bimolecular gas-phase reaction:
A2(g) + B2(g)-+ 2AB
In order for the reaction to occur, A2(g) must collide with Bz(~).
+ Rate oc frequency of the collisions, 2 (number of collisions per
second)
Rate = c,Z, where c, is a constant of proportionality.
But, 2 itself is proportional to the concentration of both A2 and B2.
i.e. 2 oc [A21 and 2 oc [B2]
Z oc [A2][B2] = ZO[A2][B2], where 2, is another constant of propor-
tionality.
* I Rate = c,ZO[A2][B2] 1