Page 150 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 150
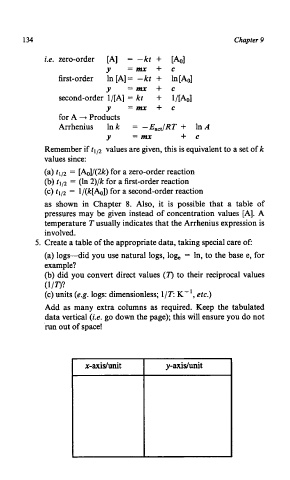
134 Chapter 9
i.e. zero-order [A] = -kt + [&I
y =mx + c
first-order In [A] = - kt + In [Ao]
y =mx + c
second-order 1/[A] = kt + l/[Ao]
y =mx+c
for A + Products
Arrhenius In k = -Eact/RT + In A
Y = mx + c
Remember if tlI2 values are given, this is equivalent to a set of k
values since:
(a) tl/2 = [&]/(2k) for a zero-order reaction
(b) tl/2 = (In 2)/k for a first-order reaction
(c) fl/2 = l/(k[&]) for a second-order reaction
as shown in Chapter 8. Also, it is possible that a table of
pressures may be given instead of concentration values [A]. A
temperature T usually indicates that the Arrhenius expression is
involved.
5. Create a table of the appropriate data, taking special care of:
(a) logs-did you use natural logs, log, = In, to the base e, for
example?
(b) did you convert direct values (7') to their reciprocal values
(1/T)?
(c) units (e.g. logs: dimensionless; 1/T: K-', etc.)
Add as many extra columns as required. Keep the tabulated
data vertical (i.e. go down the page); this will ensure you do not
run out of space!
x-axisluni t y -axidunit