Page 85 - A Working Method Approach For Introductory Physical Chemistry Calculations
P. 85
Electrochemistry I: Galvanic Cells 69
Zn(s)JZn2+(as) etc., where the short vertical line represents a phase
boundary or junction.
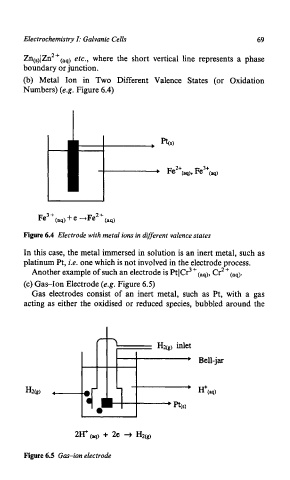
(b) Metal Ion in Two Different Valence States (or Oxidation
Numbers) (e.g. Figure 6.4)
Figure 6.4 Electrode with metal ions in different valence states
In this case, the metal immersed in solution is an inert metal, such as
platinum Pt, i.e. one which is not involved in the electrode process.
Another example of such an electrode is PtICr3+(aq), C?+(aq).
(c) Gas-Ion Electrode (e.g. Figure 6.5)
Gas electrodes consist of an inert metal, such as Pt, with a gas
acting as either the oxidised or reduced species, bubbled around the
Bell-jar
H+W
2&,) + 2e + H2(g)
Figure 6.5 Gas-ion electrode