Page 88 - A Working Method Approach For Introductory Physical Chemistry Calculations
P. 88
72 Chapter 6
The electrode used as the reference electrode is the standard hydrogen
electrode (SHE), and is assigned a potential of zero volts. The
potentials of all other electrodes are quoted relative to the standard
hydrogen electrode. This is similar to the idea of considering sea-level
as zero elevation, and then expressing all heights relative to this level:
T
+ve E" cu + 0.34 V
SHE 0.00 v
-ve E" -0.76 V
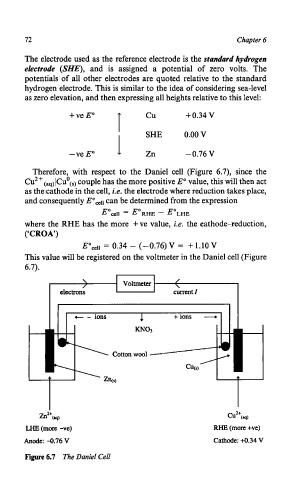
Therefore, with respect to the Daniel cell (Figure 6.7), since the
CU*+(~&U~(~) couple has the more positive E" value, this will then act
as thecathode in the cell, i.e. the electrode where reduction takes place,
and consequently Eocell can be determined from the expression
Eoceii = E'RHE - E'LHE
where the RHE has the more +ve value, i.e. the cathode-reduction,
(CCROA')
Eoce.l = 0.34 - (-0.76) V = + 1.10 V
This value will be registered on the voltmeter in the Daniel cell (Figure
6.7).
Voltmeter / i
h2+W cu2+w
LHE(morc -vc) RHE (more +ve)
Anode: -0.76V Cathode: M.34 V
Figure 6.7 The Daniel Cell