Page 105 - Adsorbents - fundamentals and applications
P. 105
90 ACTIVATED CARBON
30
20
a/mmol−g −1
10
0 0.2 0.4 0.6 0.8 1.0
p/p o
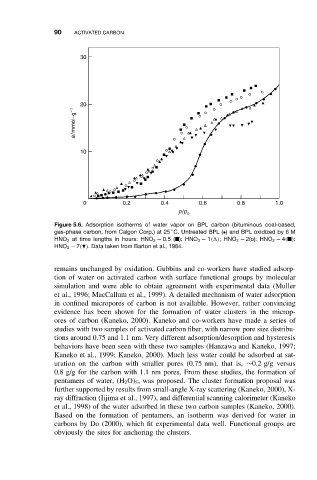
Figure 5.6. Adsorption isotherms of water vapor on BPL carbon (bituminous coal-based,
◦
gas-phase carbon, from Calgon Corp.) at 25 C. Untreated BPL (•)and BPL oxidizedby 6 M
HNO 3 at time lengths in hours: HNO 3 − 0.5( ); HNO 3 − 1( ); HNO 3 − 2(o); HNO 3 − 4( );
HNO 3 − 7( ). Data taken from Barton et al., 1984.
remains unchanged by oxidation. Gubbins and co-workers have studied adsorp-
tion of water on activated carbon with surface functional groups by molecular
simulation and were able to obtain agreement with experimental data (Muller
et al., 1996; MacCallum et al., 1999). A detailed mechanism of water adsorption
in confined micropores of carbon is not available. However, rather convincing
evidence has been shown for the formation of water clusters in the microp-
ores of carbon (Kaneko, 2000). Kaneko and co-workers have made a series of
studies with two samples of activated carbon fiber, with narrow pore size distribu-
tions around 0.75 and 1.1 nm. Very different adsorption/desorption and hysteresis
behaviors have been seen with these two samples (Hanzawa and Kaneko, 1997;
Kaneko et al., 1999; Kaneko, 2000). Much less water could be adsorbed at sat-
uration on the carbon with smaller pores (0.75 nm), that is, ∼0.2 g/g versus
0.8 g/g for the carbon with 1.1 nm pores. From these studies, the formation of
pentamers of water, (H 2 O) 5 , was proposed. The cluster formation proposal was
further supported by results from small-angle X-ray scattering (Kaneko, 2000), X-
ray diffraction (Iijima et al., 1997), and differential scanning calorimeter (Kaneko
et al., 1998) of the water adsorbed in these two carbon samples (Kaneko, 2000).
Based on the formation of pentamers, an isotherm was derived for water in
carbons by Do (2000), which fit experimental data well. Functional groups are
obviously the sites for anchoring the clusters.