Page 109 - Adsorbents - fundamentals and applications
P. 109
94 ACTIVATED CARBON
Generally, low solubility favors adsorption. The dramatic effects of the solute–
solvent interaction on adsorption may be illustrated by adsorption of aqueous
solutions of thiolane and its derivative, sulfolane. Sulfolane is of interest because
it has been used for more than 40 years for solvent extraction in a number
of industrial processes (e.g., the Sulfinol process for CO 2 removal, the UOP
Sulfolane process for recovery of aromatics from hydrocarbons, processes for
extraction of fatty acids, etc.). It has now become a serious pollutant for ground
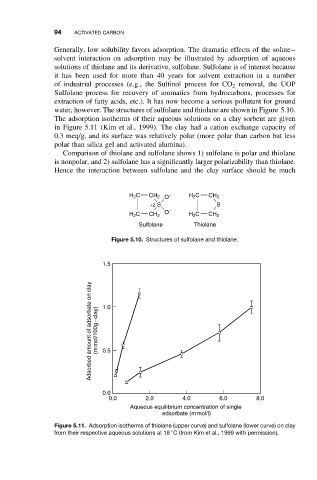
water, however. The structures of sulfolane and thiolane are shown in Figure 5.10.
The adsorption isotherms of their aqueous solutions on a clay sorbent are given
in Figure 5.11 (Kim et al., 1999). The clay had a cation exchange capacity of
0.3 meq/g, and its surface was relatively polar (more polar than carbon but less
polar than silica gel and activated alumina).
Comparison of thiolane and sulfolane shows 1) sulfolane is polar and thiolane
is nonpolar, and 2) sulfolane has a significantly larger polarizability than thiolane.
Hence the interaction between sulfolane and the clay surface should be much
H 2 C CH 2 O − H 2 C CH 2
+2 S S
H 2 C CH 2 O − H 2 C CH 2
Sulfolane Thiolane
Figure 5.10. Structures of sulfolane and thiolane.
1.5
Adsorbed amount of adsorbate on clay (mmol/100g−clay) 1.0
0.5
0.0
0.0 2.0 4.0 6.0 8.0
Aqueous equilibrium concentration of single
adsorbate (mmol/l)
Figure 5.11. Adsorption isotherms of thiolane (upper curve) and sulfolane (lower curve) on clay
◦
from their respective aqueous solutions at 18 C (from Kim et al., 1999 with permission).