Page 106 - Adsorbents - fundamentals and applications
P. 106
SURFACE CHEMISTRY AND ITS EFFECTS ON ADSORPTION 91
By more intense oxidation of the carbon, the amount of water vapor adsorbed
◦
at low relative pressures (<4Torr at 25 C) can be drastically increased. For
example, Walker and co-workers showed a 100-fold increase in water vapor
adsorption by activated carbon after strong surface oxidation by HNO 3 (Mahajan
et al., 1982). Exchange of the surface H-ions by cations (Li, Na, K, Ca) on the
oxidized carbon further increased the moisture capacity at low vapor pressures to
amounts comparable with that on zeolites. The ion-exchanged carbon was fully
◦
◦
regenerated at 140 C, in contrast to temperatures >350 C that are required for
zeolite regeneration (Mahajan et al., 1982).
As mentioned above, the surface groups can also be removed by thermal des-
orption. As expected, less water and polar molecules would be adsorbed on the
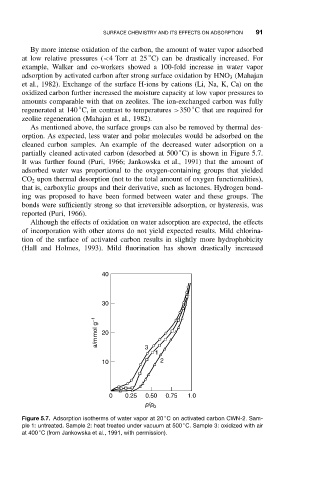
cleaned carbon samples. An example of the decreased water adsorption on a
◦
partially cleaned activated carbon (desorbed at 500 C) is shown in Figure 5.7.
It was further found (Puri, 1966; Jankowska et al., 1991) that the amount of
adsorbed water was proportional to the oxygen-containing groups that yielded
CO 2 upon thermal desorption (not to the total amount of oxygen functionalities),
that is, carboxylic groups and their derivative, such as lactones. Hydrogen bond-
ing was proposed to have been formed between water and these groups. The
bonds were sufficiently strong so that irreversible adsorption, or hysteresis, was
reported (Puri, 1966).
Although the effects of oxidation on water adsorption are expected, the effects
of incorporation with other atoms do not yield expected results. Mild chlorina-
tion of the surface of activated carbon results in slightly more hydrophobicity
(Hall and Holmes, 1993). Mild fluorination has shown drastically increased
40
30
a/mmol g −1 20
3
1
10 2
0 0.25 0.50 0.75 1.0
p/p
0
◦
Figure 5.7. Adsorption isotherms of water vapor at 20 C on activated carbon CWN-2. Sam-
◦
ple 1: untreated. Sample 2: heat treated under vacuum at 500 C. Sample 3: oxidized with air
◦
at 400 C (from Jankowska et al., 1991, with permission).