Page 102 - Adsorbents fundamentals and applications
P. 102
SURFACE CHEMISTRY AND ITS EFFECTS ON ADSORPTION 87
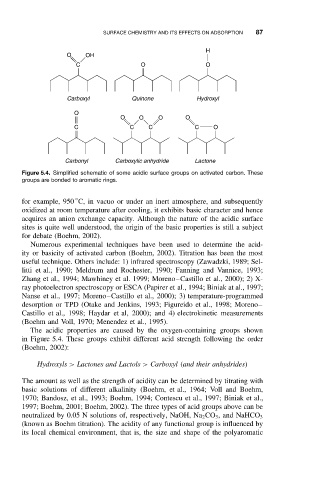
H
O OH
C O O
Carboxyl Quinone Hydroxyl
O
O O O O
C C C C O
Carbonyl Carboxylic anhydride Lactone
Figure 5.4. Simplified schematic of some acidic surface groups on activated carbon. These
groups are bonded to aromatic rings.
◦
for example, 950 C, in vacuo or under an inert atmosphere, and subsequently
oxidized at room temperature after cooling, it exhibits basic character and hence
acquires an anion exchange capacity. Although the nature of the acidic surface
sites is quite well understood, the origin of the basic properties is still a subject
for debate (Boehm, 2002).
Numerous experimental techniques have been used to determine the acid-
ity or basicity of activated carbon (Boehm, 2002). Titration has been the most
useful technique. Others include: 1) infrared spectroscopy (Zawadzki, 1989; Sel-
litti et al., 1990; Meldrum and Rochester, 1990; Fanning and Vannice, 1993;
Zhang et al., 1994; Mawhiney et al. 1999; Moreno–Castillo et al., 2000); 2) X-
ray photoelectron spectroscopy or ESCA (Papirer et al., 1994; Biniak at al., 1997;
Nanse et al., 1997; Moreno–Castillo et al., 2000); 3) temperature-programmed
desorption or TPD (Otake and Jenkins, 1993; Figureido et al., 1998; Moreno–
Castillo et al., 1998; Haydar et al, 2000); and 4) electrokinetic measurements
(Boehm and Voll, 1970; Menendez et al., 1995).
The acidic properties are caused by the oxygen-containing groups shown
in Figure 5.4. These groups exhibit different acid strength following the order
(Boehm, 2002):
Hydroxyls > Lactones and Lactols > Carboxyl (and their anhydrides)
The amount as well as the strength of acidity can be determined by titrating with
basic solutions of different alkalinity (Boehm, et al., 1964; Voll and Boehm,
1970; Bandosz, et al., 1993; Boehm, 1994; Contescu et al., 1997; Biniak et al.,
1997; Boehm, 2001; Boehm, 2002). The three types of acid groups above can be
neutralized by 0.05 N solutions of, respectively, NaOH, Na 2 CO 3 , and NaHCO 3
(known as Boehm titration). The acidity of any functional group is influenced by
its local chemical environment, that is, the size and shape of the polyaromatic