Page 99 - Adsorbents fundamentals and applications
P. 99
84 ACTIVATED CARBON
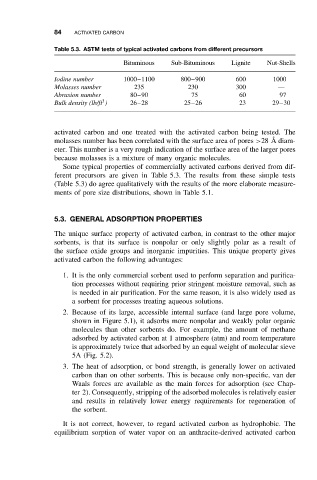
Table 5.3. ASTM tests of typical activated carbons from different precursors
Bituminous Sub-Bituminous Lignite Nut-Shells
Iodine number 1000–1100 800–900 600 1000
Molasses number 235 230 300 —
Abrasion number 80–90 75 60 97
3
Bulk density (lb/ft ) 26–28 25–26 23 29–30
activated carbon and one treated with the activated carbon being tested. The
molasses number has been correlated with the surface area of pores >28 ˚ Adiam-
eter. This number is a very rough indication of the surface area of the larger pores
because molasses is a mixture of many organic molecules.
Some typical properties of commercially activated carbons derived from dif-
ferent precursors are given in Table 5.3. The results from these simple tests
(Table 5.3) do agree qualitatively with the results of the more elaborate measure-
ments of pore size distributions, shown in Table 5.1.
5.3. GENERAL ADSORPTION PROPERTIES
The unique surface property of activated carbon, in contrast to the other major
sorbents, is that its surface is nonpolar or only slightly polar as a result of
the surface oxide groups and inorganic impurities. This unique property gives
activated carbon the following advantages:
1. It is the only commercial sorbent used to perform separation and purifica-
tion processes without requiring prior stringent moisture removal, such as
is needed in air purification. For the same reason, it is also widely used as
a sorbent for processes treating aqueous solutions.
2. Because of its large, accessible internal surface (and large pore volume,
shown in Figure 5.1), it adsorbs more nonpolar and weakly polar organic
molecules than other sorbents do. For example, the amount of methane
adsorbed by activated carbon at 1 atmosphere (atm) and room temperature
is approximately twice that adsorbed by an equal weight of molecular sieve
5A (Fig. 5.2).
3. The heat of adsorption, or bond strength, is generally lower on activated
carbon than on other sorbents. This is because only non-specific, van der
Waals forces are available as the main forces for adsorption (see Chap-
ter 2). Consequently, stripping of the adsorbed molecules is relatively easier
and results in relatively lower energy requirements for regeneration of
the sorbent.
It is not correct, however, to regard activated carbon as hydrophobic. The
equilibrium sorption of water vapor on an anthracite-derived activated carbon