Page 174 - Adsorbents fundamentals and applications
P. 174
ZEOLITE TYPESA,X,AND Y 159
(a) (b) (c)
II
3 1 4 II′
4
III′ III
2 4
1
III
II
I
(d) (e)
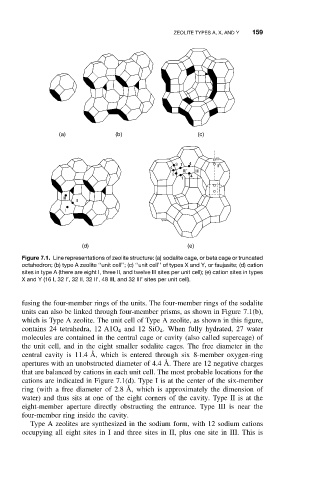
Figure 7.1. Line representations of zeolite structure: (a) sodalite cage, or beta cage or truncated
octahedron; (b) type A zeolite ‘‘unit cell’’; (c) ‘‘unit cell’’ of types X and Y, or faujasite; (d) cation
sites in type A (there are eight I, three II, and twelve III sites per unit cell); (e) cation sites in types
X and Y (16 I, 32 I’, 32 II, 32 II’, 48 III, and 32 III’ sites per unit cell).
fusing the four-member rings of the units. The four-member rings of the sodalite
units can also be linked through four-member prisms, as shown in Figure 7.1(b),
which is Type A zeolite. The unit cell of Type A zeolite, as shown in this figure,
contains 24 tetrahedra, 12 A1O 4 and 12 SiO 4 . When fully hydrated, 27 water
molecules are contained in the central cage or cavity (also called supercage) of
the unit cell, and in the eight smaller sodalite cages. The free diameter in the
central cavity is 11.4 ˚ A, which is entered through six 8-member oxygen-ring
apertures with an unobstructed diameter of 4.4 ˚ A. There are 12 negative charges
that are balanced by cations in each unit cell. The most probable locations for the
cations are indicated in Figure 7.1(d). Type I is at the center of the six-member
ring (with a free diameter of 2.8 ˚ A, which is approximately the dimension of
water) and thus sits at one of the eight corners of the cavity. Type II is at the
eight-member aperture directly obstructing the entrance. Type III is near the
four-member ring inside the cavity.
Type A zeolites are synthesized in the sodium form, with 12 sodium cations
occupying all eight sites in I and three sites in II, plus one site in III. This is