Page 176 - Adsorbents fundamentals and applications
P. 176
ZEOLITE TYPESA,X,AND Y 161
7.1.3. Examples of Molecular Sieving
Separation and purification can be accomplished by molecular sieving, that is, by
size exclusion. An example is drying or dehydration of gases or alcohols by 3A
zeolite, which excludes all hydrocarbons, O 2 ,N 2 , and essentially all permanent
gases except ammonia. It is particularly useful for drying gases under reactive
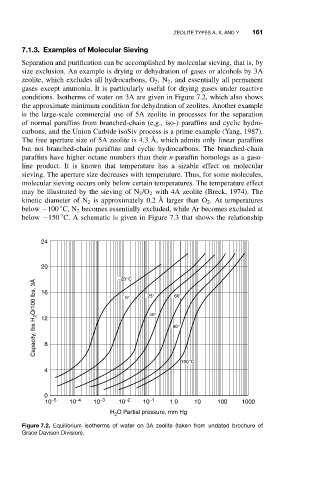
conditions. Isotherms of water on 3A are given in Figure 7.2, which also shows
the approximate minimum condition for dehydration of zeolites. Another example
is the large-scale commercial use of 5A zeolite in processes for the separation
of normal paraffins from branched-chain (e.g., iso-) paraffins and cyclic hydro-
carbons, and the Union Carbide isoSiv process is a prime example (Yang, 1987).
The free aperture size of 5A zeolite is 4.3 ˚ A, which admits only linear paraffins
but not branched-chain paraffins and cyclic hydrocarbons. The branched-chain
paraffins have higher octane numbers than their n-paraffin homologs as a gaso-
line product. It is known that temperature has a sizable effect on molecular
sieving. The aperture size decreases with temperature. Thus, for some molecules,
molecular sieving occurs only below certain temperatures. The temperature effect
may be illustrated by the sieving of N 2 /O 2 with 4A zeolite (Breck, 1974). The
kinetic diameter of N 2 is approximately 0.2 ˚ A larger than O 2 . At temperatures
◦
below −100 C, N 2 becomes essentially excluded, while Ar becomes excluded at
◦
below −150 C. A schematic is given in Figure 7.3 that shows the relationship
24
20
−20 °C 25° 60°
Capacity, lbs H 2 O/100 lbs, 3Å 12 40° 80°
16
0°
8
100 °C
4
0
10 −5 10 −4 10 −3 10 −2 10 −1 1.0 10 100 1000
H O Partial pressure, mm Hg
2
Figure 7.2. Equilibrium isotherms of water on 3A zeolite (taken from undated brochure of
Grace Davison Division).