Page 212 - Adsorbents fundamentals and applications
P. 212
PREPARATION OF THREE TYPES OF SORBENTS 197
800
600
Intensity (a.u.) 400 Temp. (°C)
200
0
0 20 40 60
Time (min.)
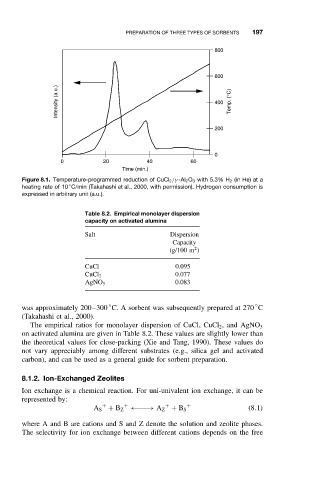
Figure 8.1. Temperature-programmed reduction of CuCl 2 /γ -Al 2 O 3 with 5.3% H 2 (in He) at a
◦
heating rate of 10 C/min (Takahashi et al., 2000, with permission). Hydrogen consumption is
expressed in arbitrary unit (a.u.).
Table 8.2. Empirical monolayer dispersion
capacity on activated alumina
Salt Dispersion
Capacity
2
(g/100 m )
CuCl 0.095
CuCl 2 0.077
0.083
AgNO 3
◦
◦
was approximately 200–300 C. A sorbent was subsequently prepared at 270 C
(Takahashi et al., 2000).
The empirical ratios for monolayer dispersion of CuCl, CuCl 2 , and AgNO 3
on activated alumina are given in Table 8.2. These values are slightly lower than
the theoretical values for close-packing (Xie and Tang, 1990). These values do
not vary appreciably among different substrates (e.g., silica gel and activated
carbon), and can be used as a general guide for sorbent preparation.
8.1.2. Ion-Exchanged Zeolites
Ion exchange is a chemical reaction. For uni-univalent ion exchange, it can be
represented by:
+ + + +
A S + B Z ←−−( A Z + B S (8.1)
where A and B are cations and S and Z denote the solution and zeolite phases.
The selectivity for ion exchange between different cations depends on the free