Page 260 - Adsorbents fundamentals and applications
P. 260
CARBON NANOTUBES 245
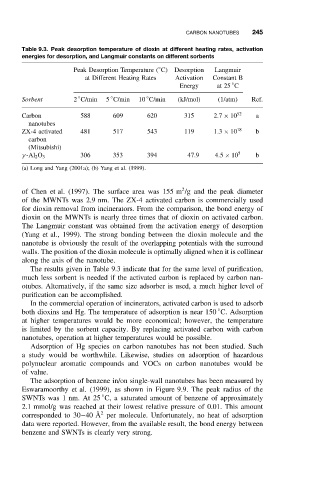
Table 9.3. Peak desorption temperature of dioxin at different heating rates, activation
energies for desorption, and Langmuir constants on different sorbents
◦
Peak Desorption Temperature ( C) Desorption Langmuir
at Different Heating Rates Activation Constant B
◦
Energy at 25 C
◦
◦
◦
Sorbent 2 C/min 5 C/min 10 C/min (kJ/mol) (1/atm) Ref.
Carbon 588 609 620 315 2.7 × 10 52 a
nanotubes
ZX-4 activated 481 517 543 119 1.3 × 10 18 b
carbon
(Mitsubishi)
306 353 394 47.9 4.5 × 10 5 b
γ -Al 2 O 3
(a) Long and Yang (2001a); (b) Yang et al. (1999).
2
of Chen et al. (1997). The surface area was 155 m /g and the peak diameter
of the MWNTs was 2.9 nm. The ZX-4 activated carbon is commercially used
for dioxin removal from incinerators. From the comparison, the bond energy of
dioxin on the MWNTs is nearly three times that of dioxin on activated carbon.
The Langmuir constant was obtained from the activation energy of desorption
(Yang et al., 1999). The strong bonding between the dioxin molecule and the
nanotube is obviously the result of the overlapping potentials with the surround
walls. The position of the dioxin molecule is optimally aligned when it is collinear
along the axis of the nanotube.
The results given in Table 9.3 indicate that for the same level of purification,
much less sorbent is needed if the activated carbon is replaced by carbon nan-
otubes. Alternatively, if the same size adsorber is used, a much higher level of
purification can be accomplished.
In the commercial operation of incinerators, activated carbon is used to adsorb
◦
both dioxins and Hg. The temperature of adsorption is near 150 C. Adsorption
at higher temperatures would be more economical; however, the temperature
is limited by the sorbent capacity. By replacing activated carbon with carbon
nanotubes, operation at higher temperatures would be possible.
Adsorption of Hg species on carbon nanotubes has not been studied. Such
a study would be worthwhile. Likewise, studies on adsorption of hazardous
polynuclear aromatic compounds and VOCs on carbon nanotubes would be
of value.
The adsorption of benzene in/on single-wall nanotubes has been measured by
Eswaramoorthy et al. (1999), as shown in Figure 9.9. The peak radius of the
◦
SWNTs was 1 nm. At 25 C, a saturated amount of benzene of approximately
2.1 mmol/g was reached at their lowest relative pressure of 0.01. This amount
2
corresponded to 30–40 ˚ A per molecule. Unfortunately, no heat of adsorption
data were reported. However, from the available result, the bond energy between
benzene and SWNTs is clearly very strong.