Page 332 - Adsorbents fundamentals and applications
P. 332
HYDROGEN STORAGE 317
The first step in examining such possibility is to see whether H atoms can
adsorb on the basal plane of graphite. This would involve electron transfer or
formation of a chemical bond. Monte Carlo simulation cannot be used for this
study; rather, molecular orbital theory calculations can provide a full description
of the process (Chapter 8). Molecular orbital calculations on the chemisorption
of atomic hydrogen on graphite have been reported since the early development
of quantum chemistry, that is, the calculations of H/graphite by Sherman and
Eyring (1932). The subsequent calculations on H/graphite have been mostly per-
formed with semi-empirical methods. Chen and Yang (1989) performed such
calculations and showed that the chemisorption was most stable on the zigzag
edges, followed closely by the armchair edges, while the adsorption on the basal
plane was meta-stable. More recently, Yang and Yang (2002) have undertaken
ab initio calculations using Gaussian. The ab initio method is capable of energy
predictions. Their results are summarized below.
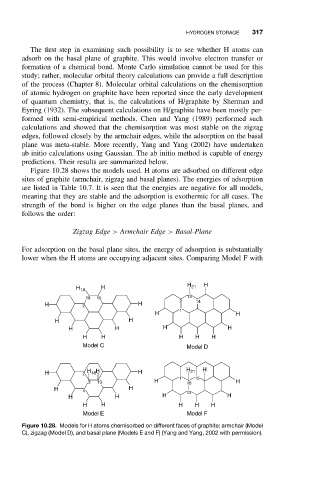
Figure 10.28 shows the models used. H atoms are adsorbed on different edge
sites of graphite (armchair, zigzag and basal planes). The energies of adsorption
are listed in Table 10.7. It is seen that the energies are negative for all models,
meaning that they are stable and the adsorption is exothermic for all cases. The
strength of the bond is higher on the edge planes than the basal planes, and
follows the order:
Zigzag Edge > Armchair Edge > Basal-Plane
For adsorption on the basal plane sites, the energy of adsorption is substantially
lower when the H atoms are occupying adjacent sites. Comparing Model F with
H H
H 18 H 21
16 15 13
H 2 H 2 14
1
H H
H H
H H H H
H H H H H
Model C Model D
H 2 H H H H 21 H
18
1 15
3 13 H 16 H
H 4 H 12
H H H H
H H H H H
Model E Model F
Figure 10.28. Models for H atoms chemisorbed on different faces of graphite: armchair (Model
C), zigzag (Model D), and basal plane (Models E and F) (Yang and Yang, 2002 with permission).