Page 334 - Adsorbents fundamentals and applications
P. 334
HYDROGEN STORAGE 319
Lee and Lee (2000) performed density-functional calculations for hydrogen
chemisorption in SMNT, both inside and outside the tube. Their calculations
were performed at zero Kelvin and showed two configurations for chemisorption.
They predicted that 14 wt % could be adsorbed in (10,10) tubes.
Multi-wall Nanotubes. The platelet-structure of GNF is described in a pre-
ceding section. When the angle between the platelets and the fiber axis is small,
there is little or no distinction between MWNTs and GNF. Both are grown cat-
alytically, and the TEM images of these two types of materials can be essentially
the same. In fact, it is known that the surfaces of the vapor-grown MWNTs are
rarely perfect graphite planes and that their surfaces have functionalities (e.g.,
Kuznetsova et al., 2000).
It is these materials on which the widest range of hydrogen capacities have
been reported. The pretreatment condition and the residual catalyst are clearly
important in the hydrogen capacity, and yet these have not been clearly char-
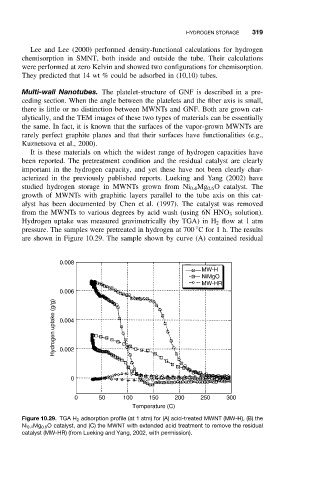
acterized in the previously published reports. Lueking and Yang (2002) have
studied hydrogen storage in MWNTs grown from Ni 0.4 Mg 0.6 O catalyst. The
growth of MWNTs with graphitic layers parallel to the tube axis on this cat-
alyst has been documented by Chen et al. (1997). The catalyst was removed
from the MWNTs to various degrees by acid wash (using 6N HNO 3 solution).
Hydrogen uptake was measured gravimetrically (by TGA) in H 2 flow at 1 atm
◦
pressure. The samples were pretreated in hydrogen at 700 C for 1 h. The results
are shown in Figure 10.29. The sample shown by curve (A) contained residual
0.008
MW-H
NiMgO
MW-HR
0.006
Hydrogen uptake (g/g) 0.004
0.002
0
0 50 100 150 200 250 300
Temperature (C)
Figure 10.29. TGA H 2 adsorption profile (at 1 atm) for (A) acid-treated MWNT (MW-H), (B) the
Ni 0.4 Mg 0.6 O catalyst, and (C) the MWNT with extended acid treatment to remove the residual
catalyst (MW-HR) (from Lueking and Yang, 2002, with permission).