Page 338 - Adsorbents fundamentals and applications
P. 338
METHANE STORAGE 323
A high packing density is required for high V/V storage. A clever approach
for achieving high packing density is to form monoliths (such as discs) by using
a polymeric binder (Bose et al., 1991). Typically, the sorbent powder is mixed
with a binder and the mixture is moulded into discs under a high pressure. The
◦
monoliths are subsequently produced upon heat-treatment (e.g., 800 CinN 2 )
(Chen and McEnaney, 1995). The percentages of binder shown in Table 10.8 are
the optimal amounts for maximum V/V methane storage.
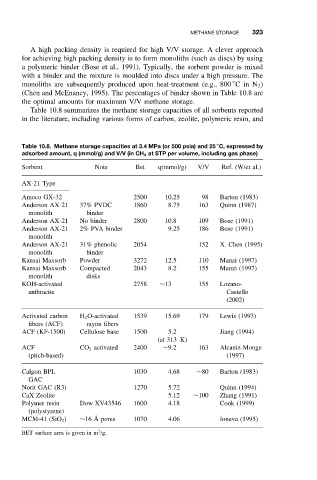
Table 10.8 summarizes the methane storage capacities of all sorbents reported
in the literature, including various forms of carbon, zeolite, polymeric resin, and
◦
Table 10.8. Methane storage capacities at 3.4 MPa (or 500 psia) and 25 C, expressed by
adsorbed amount, q (mmol/g) and V/V (in CH 4 at STP per volume, including gas phase)
Sorbent Note Bet q(mmol/g) V/V Ref. (W/et al.)
AX-21 Type
Amoco GX-32 2500 10.25 98 Barton (1983)
Anderson AX-21 37% PVDC 1860 8.75 163 Quinn (1987)
monolith binder
Anderson AX-21 No binder 2800 10.8 109 Bose (1991)
Anderson AX-21 2% PVA binder 9.25 186 Bose (1991)
monolith
Anderson AX-21 31% phenolic 2054 152 X. Chen (1995)
monolith binder
Kansai Maxsorb Powder 3272 12.5 110 Manzi (1997)
Kansai Maxsorb Compacted 2043 8.2 155 Manzi (1997)
monolith disks
KOH-activated 2758 ∼13 155 Lozano-
anthracite Castello
(2002)
Activated carbon H 2 O-activated 1539 15.69 179 Lewis (1993)
fibers (ACF) rayon fibers
ACF (KF-1500) Cellulose base 1500 5.2 Jiang (1994)
(at 313 K)
ACF CO 2 activated 2400 ∼9.2 163 Alcaniz-Monge
(pitch-based) (1997)
Calgon BPL 1030 4.68 ∼80 Barton (1983)
GAC
Norit GAC (R3) 1270 5.72 Quinn (1994)
CaX Zeolite 5.12 ∼100 Zhang (1991)
Polymer resin Dow XV43546 1600 4.18 Cook (1999)
(polystyrene)
MCM-41 (SiO 2 ) ∼16 ˚ A pores 1070 4.06 Ioneva (1995)
2
BET surface area is given in m /g.