Page 342 - Adsorbents fundamentals and applications
P. 342
OLEFIN/PARAFFIN SEPARATIONS 327
former type may be used for kinetic separation, that is, based on the higher diffu-
sivities of olefins over that of paraffins. The use of 4A zeolite (Ramachandran and
Dao, 1994) is one such example. AlPO 4 -14 molecular sieve (Padin et al., 2000)
is another possibility. Syntheses and characterizations of the π-complexation sor-
bents for olefin/paraffin separations have been discussed in Chapter 8 (8.3 and
8.4). The aluminophosphates (AlPO 4 s) and silicoaluminophosphates (SAPO 4 s)
are discussed in Chapter 7 (7.2.2).
A general comparison can be made of these two types of sorbents. The adsorp-
tion of olefins under practical conditions is limited by the pore volume of the
sorbent, that is, adsorption at pressures above ambient or adsorption at near
room temperature and atmospheric pressure. The pore volumes of zeolites and
molecular sieves are substantially lower than that of the π-complexation sor-
bents, which are based on silica gel and activated alumina. Thus, for propylene,
the limiting adsorbed amounts for zeolites and molecular sieves are approxi-
mately 2.1–2.4 mmol/g, whereas that for the π-complexation sorbents supported
on silica gel is well over 5 mmol/g. As will be shown below, direct compar-
isons of the PSA separation performances with these two types of sorbents show
indeed that the π-complexation sorbents are significantly better than zeolites and
molecular sieves.
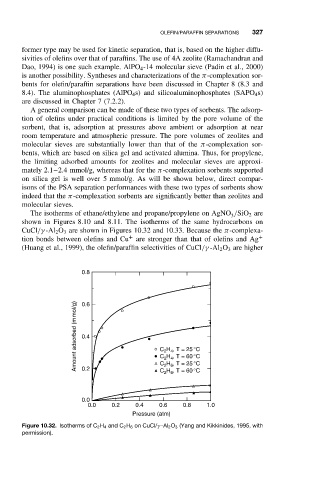
The isotherms of ethane/ethylene and propane/propylene on AgNO /SiO 2 are
3
shown in Figures 8.10 and 8.11. The isotherms of the same hydrocarbons on
CuCl/γ -Al 2 O 3 are shown in Figures 10.32 and 10.33. Because the π-complexa-
+
tion bonds between olefins and Cu are stronger than that of olefins and Ag +
(Huang et al., 1999), the olefin/paraffin selectivities of CuCl/γ -Al 2 O 3 are higher
0.8
Amount adsorbed (mmol/g) 0.4 C H , T = 25 °C
0.6
2 4
C H , T = 60 °C
2 4
C H , T = 25 °C
2 6
0.2
2 6
0.0 C H , T = 60 °C
0.0 0.2 0.4 0.6 0.8 1.0
Pressure (atm)
Figure 10.32. Isotherms of C 2 H 4 and C 2 H 6 on CuCl/γ -Al 2 O 3 (Yang and Kikkinides, 1995, with
permission).