Page 345 - Adsorbents fundamentals and applications
P. 345
330 SORBENTS FOR APPLICATIONS
and throughput. A fair comparison of sorbent performance can be made by using
similar cycle conditions for each of the sorbent systems and by comparing one of
the performance parameters with the other two parameters being kept nearly the
same. It should be noted that the term “product” refers to the C 3 H 6 -rich effluent
obtained in the desorption step (4). The various process variables were defined
as follows:
C 3H 6 from C 3H 6 used for purging
step 4 − in step 3
Product recovery = (10.4)
(C 3 H 6 fed instep1and step2)
(C 3 H 6 used to purge in step 3)
Purge/feed ratio (P/F) = (10.5)
(C 3 H 6 fed in step1 andstep2)
Another important parameter used to gauge the adsorbent’s productivity is the
product throughput:
Amount (kg) of C H 6 product per hour
3
Product throughput = (10.6)
Amount (kg) of adsorbent
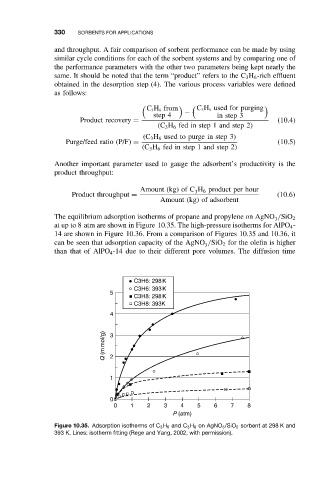
The equilibrium adsorption isotherms of propane and propylene on AgNO /SiO 2
3
at up to 8 atm are shown in Figure 10.35. The high-pressure isotherms for AlPO 4 -
14 are shown in Figure 10.36. From a comparison of Figures 10.35 and 10.36, it
can be seen that adsorption capacity of the AgNO /SiO 2 for the olefin is higher
3
than that of AlPO 4 -14 due to their different pore volumes. The diffusion time
C3H6: 298K
C3H6: 393K
5
C3H8: 298K
C3H8: 393K
4
Q (m mol/g) 3
2
1
0
0 1 2 3 4 5 6 7 8
P (atm)
Figure 10.35. Adsorption isotherms of C 3 H 6 and C 3 H 8 on AgNO 3 /SiO 2 sorbent at 298 K and
393 K. Lines: isotherm fitting (Rege and Yang, 2002, with permission).