Page 343 - Adsorbents fundamentals and applications
P. 343
328 SORBENTS FOR APPLICATIONS
0.8
Amount adsorbed (mmol/g) 0.6 C 3 H 8 , T = 25°C
0.4
C 3 H 6 , T = 25°C
C H , T = 60°C
3 8
C H , T = 60 °C
3 6
0.2
0.0
0.0 0.2 0.4 0.6 0.8 1.0
Pressure (atm)
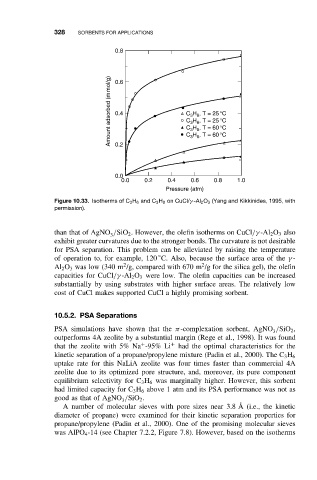
Figure 10.33. Isotherms of C 3 H 6 and C 3 H 8 on CuCl/γ -Al 2 O 3 (Yang and Kikkinides, 1995, with
permission).
than that of AgNO /SiO 2 . However, the olefin isotherms on CuCl/γ -Al 2 O 3 also
3
exhibit greater curvatures due to the stronger bonds. The curvature is not desirable
for PSA separation. This problem can be alleviated by raising the temperature
◦
of operation to, for example, 120 C. Also, because the surface area of the γ -
2
2
Al 2 O 3 was low (340 m /g, compared with 670 m /g for the silica gel), the olefin
capacities for CuCl/γ -Al 2 O 3 were low. The olefin capacities can be increased
substantially by using substrates with higher surface areas. The relatively low
cost of CuCl makes supported CuCl a highly promising sorbent.
10.5.2. PSA Separations
PSA simulations have shown that the π-complexation sorbent, AgNO /SiO 2 ,
3
outperforms 4A zeolite by a substantial margin (Rege et al., 1998). It was found
+
that the zeolite with 5% Na -95% Li + had the optimal characteristics for the
kinetic separation of a propane/propylene mixture (Padin et al., 2000). The C 3 H 6
uptake rate for this NaLiA zeolite was four times faster than commercial 4A
zeolite due to its optimized pore structure, and, moreover, its pure component
equilibrium selectivity for C 3 H 6 was marginally higher. However, this sorbent
had limited capacity for C 3 H 6 above 1 atm and its PSA performance was not as
good as that of AgNO /SiO 2 .
3
A number of molecular sieves with pore sizes near 3.8 ˚ A (i.e., the kinetic
diameter of propane) were examined for their kinetic separation properties for
propane/propylene (Padin et al., 2000). One of the promising molecular sieves
was AlPO 4 -14 (see Chapter 7.2.2, Figure 7.8). However, based on the isotherms