Page 339 - Adsorbents fundamentals and applications
P. 339
324 SORBENTS FOR APPLICATIONS
silica (MCM-41). These represent the highest reported storage capacities from
each category of sorbents. The V/V capacity is expressed in volume of methane
stored (cc STP, including gas phase) per volume of sorbent. This is not the
deliverable storage. The adsorbed amount at 1 atm needs to be subtracted to get
the delivered amount. Empirically, the delivered capacity is about 15% lower
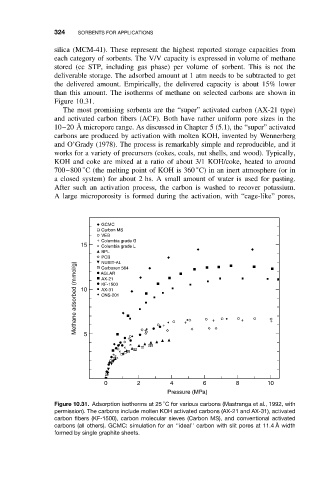
than this amount. The isotherms of methane on selected carbons are shown in
Figure 10.31.
The most promising sorbents are the “super” activated carbon (AX-21 type)
and activated carbon fibers (ACF). Both have rather uniform pore sizes in the
10–20 ˚ A micropore range. As discussed in Chapter 5 (5.1), the “super” activated
carbons are produced by activation with molten KOH, invented by Wennerberg
and O’Grady (1978). The process is remarkably simple and reproducible, and it
works for a variety of precursors (cokes, coals, nut shells, and wood). Typically,
KOH and coke are mixed at a ratio of about 3/1 KOH/coke, heated to around
◦
◦
700–800 C (the melting point of KOH is 360 C) in an inert atmosphere (or in
a closed system) for about 2 hs. A small amount of water is used for pasting.
After such an activation process, the carbon is washed to recover potassium.
A large microporosity is formed during the activation, with “cage-like” pores,
GCMC
Carbon MS
VEB
Columbia grade G
15 Columbia grade L
BPL
PCB
NUXIT-AL
Methane adsorbed (m mol/g) 10 AX-21
Carboxen 564
AGLAR
KF-1500
AX-31
CNS-201
5
0 2 4 6 8 10
Pressure (MPa)
◦
Figure 10.31. Adsorption isotherms at 25 C for various carbons (Mastranga et al., 1992, with
permission). The carbons include molten KOH activated carbons (AX-21 and AX-31), activated
carbon fibers (KF-1500), carbon molecular sieves (Carbon MS), and conventional activated
carbons (all others). GCMC: simulation for an ‘‘ideal’’ carbon with slit pores at 11.4 ˚ Awidth
formed by single graphite sheets.