Page 313 - Adsorption by Powders and Porous Solids
P. 313
ADSORPTION BY POWDERS AND POROUS SOI.IDS
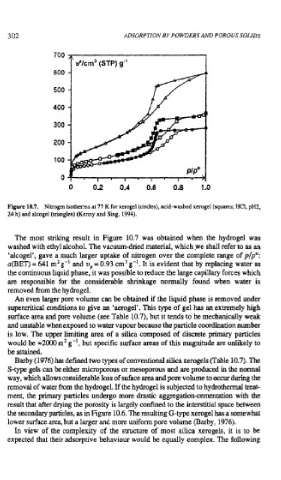
Figure 10.7. Nitrogen isotherms at 77 K for xerogel (circles), acid-washed xerogel (squares; HCl, pH2,
24 h) and alcogel (triangles) (Kenny and Sing, 1994).
The most striking result in Figure 10.7 was obtained when the hydrogel was
washed with ethyl alcohol. The vacuum-dried material, which .we shall refer to as an
'alcogel', gave a much larger uptake of nitrogen over the complete range of p/pO:
a(BET) = 641 m2 g-l and v, = 0.93 cm g -'. It is evident that by replacing water as
the continuous liquid phase, it was possible to reduce the large capillary forces which
are responsible for the considerable shrinkage normally found when water is
removed from the hydrogel.
An even larger pore volume can be obtained if the liquid phase is removed under
supercritical conditions to give an 'aerogel'. This type of gel has an extremely high
surface area and pore volume (see Table 10.7), but it tends to be mechanically weak
and unstable when exposed to water vapour because the particle coordination number
is low. The upper limiting area of a silica composed of discrete primary particles
would be ~2000 rnZ g-', but specific surface areas of this magnitude are unlikely to
be attained.
Barby (1 976) has defmed two types of conventional silica xerogels (Table 10.7). The
S-type gels can be either microporous or mesoporous and are produced in the normal
way, which allows considerable loss of suface area and pore volume to occur during the
removal of water from the hydrogel. If the hydrogel is subjected to hydrothermal treat-
ment, the primary particles undergo more drastic aggregation-cementation with the
result that after drying the porosity is largely confined to the interstitial space between
the secondary particles, as in Figure 10.6. The resulting G-type xerogel has a somewhat
lower surface area, but a larger and more uniform pore volume (Barby, 1976).
In view of the complexity of the structure of most silica xerogels, it is to be
expected that their adsorptive behaviour would be equally complex. The following