Page 29 - Adsorption Technology & Design, Elsevier (1998)
P. 29
26 Adsorbents
and the channel size is reduced to 0.38 nm. If the Ca 2+ is replaced by K §
then the larger cation reduces the channel size even further to 0.3 nm.
In addition to changes to the cationic structure, the Si/AI ratio can be varied
during manufacture from unity to well over 1000. Thus zeolites with widely
different adsorptive properties may be tailored by the appropriate choice of
framework structure, cationic form and silica to alumina ratio in order to
achieve the selectivity required for a given separation. Many zeolites are
extremely polar and therefore separations may be effected using both
molecular sieving and internal surface property effects. The kinetic selectivity is
determined from the free diameters of the windows in the intra-crystalline
channel structure. Examples of such diameters, together with the principal
properties and main uses of zeolites, are given in Table 2.4.
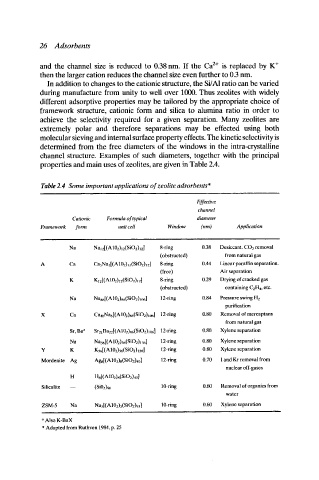
Table 2.4 Some important applications of zeolite adsorbents*
Effective
channel
diameter
Cationic Formula of typical
Framework form unit cell Window (nm) Application
0.38 Desiccant. CO2 removal
Na Na12[(A102)12(SiO2)12] 8-ring
from natural gas
(obstructed)
Linear paraffin separation.
Ca CasNa21(A102)12(SiO2)J2] 8-ring 0.44
(free) Air separation
0.29 Drying of cracked gas
K KI2[(AI02)12(SiO2)I2] 8-ring
(obstructed) containing C2H4, etc.
0.84 Pressure swing H2
Na Nasr[(A102)sr(SiO2)wr] 12-ring
purification
0.80 Removal of mercaptans
Ca Ca4oNa6[(A 102)86(SiO2)1tm] 12-ring
from natural gas
0.80 Xylene separation
Sr, Ba a Sr21Ba22[(A102)86(SiO2)106] 12-ring
0.80 Xylene separation
Na Na56[(A 102)5t,(SiO2)136] 12-ring
0.80 Xylene separation
Y K K56[(A102)56(SiO2)I36] 12-ring
0.70 I and Kr removal from
Mordenite Ag Ags[(A102)s(SiO2)4o] 12-ring
nuclear off-gases
Hs[(A102)8(SiO2)4o]
10-ring 0.60 Removal of organics from
Silicalite (Si02)96
water
10-ring 0.60 Xylene separation
ZSM-5 Na Na3[(A102)3(SiO2)93]
i
,
a Also K-BaX
* Adapted from Ruthven 1984, p. 25