Page 28 - Adsorption Technology & Design, Elsevier (1998)
P. 28
Adsorbents 25
aperture sizes of 0.42, 0.57 and 0.74 nm, respectively, and are penetrable by
molecules of increasing size. It is possible for molecules slightly larger than
the aperture size to gain access to the cavities because of the vibration of
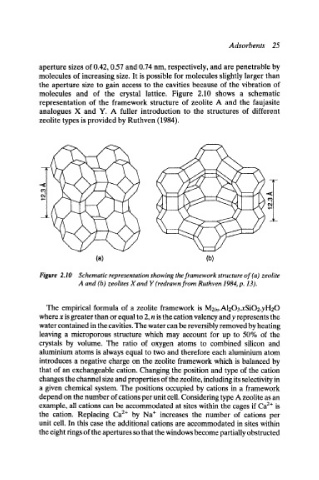
molecules and of the crystal lattice. Figure 2.10 shows a schematic
representation of the framework structure of zeolite A and the faujasite
analogues X and Y. A fuller introduction to the structures of different
zeolite types is provided by Ruthven (1984).
,<
(a) (b)
Figure 2.10 Schematic representation showing the framework structure of (a) zeolite
A and (b) zeolites Xand Y (redrawn from Ruthven 1984, p. 13).
The empirical formula of a zeolite framework is M2/n.AI203.xSiO2.yH20
where x is greater than or equal to 2, n is the cation valency and y represents the
water contained in the cavities. The water can be reversibly removed by heating
leaving a microporous structure which may account for up to 50% of the
crystals by volume. The ratio of oxygen atoms to combined silicon and
aluminium atoms is always equal to two and therefore each aluminium atom
introduces a negative charge on the zeolite framework which is balanced by
that of an exchangeable cation. Changing the position and type of the cation
changes the channel size and properties of the zeolite, including its selectivity in
a given chemical system. The positions occupied by cations in a framework
depend on the number of cations per unit cell. Considering type A zeolite as an
example, all cations can be accommodated at sites within the cages if Ca 2§ is
the cation. Replacing Ca 2+ by Na § increases the number of cations per
unit cell. In this case the additional cations are accommodated in sites within
the eight rings of the apertures so that the windows become partially obstructed