Page 36 - Adsorption Technology & Design, Elsevier (1998)
P. 36
Fundamentals of adsorption equilibria 33
10
A
'T
(9
o 1.0 r o 1.25 r o 1.5 r o
E
I ............ I __ I
v
i Distance from su~.~...~~ ]
A
t,.,
U'(r o) ~ Heat of
i adsorption
-5
i J a I
-10
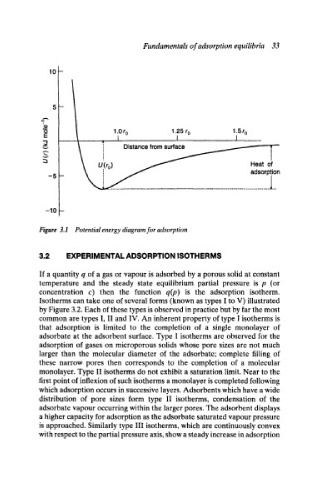
Figure 3.1 Potential energy diagram for adsorption
3.2 EXPERIMENTAL ADSORPTION ISOTHERMS
If a quantity q of a gas or vapour is adsorbed by a porous solid at constant
temperature and the steady state equilibrium partial pressure is p (or
concentration c) then the function q(p) is the adsorption isotherm.
Isotherms can take one of several forms (known as types I to V) illustrated
by Figure 3.2. Each of these types is observed in practice but by far the most
common are types I, II and IV. An inherent property of type I isotherms is
that adsorption is limited to the completion of a single monolayer of
adsorbate at the adsorbent surface. Type I isotherms are observed for the
adsorption of gases on microporous solids whose pore sizes are not much
larger than the molecular diameter of the adsorbate; complete filling of
these narrow pores then corresponds to the completion of a molecular
monolayer. Type II isotherms do not exhibit a saturation limit. Near to the
first point of inflexion of such isotherms a monolayer is completed following
which adsorption occurs in successive layers. Adsorbents which have a wide
distribution of pore sizes form type II isotherms, condensation of the
adsorbate vapour occurring within the larger pores. The adsorbent displays
a higher capacity for adsorption as the adsorbate saturated vapour pressure
is approached. Similarly type III isotherms, which are continuously convex
with respect to the partial pressure axis, show a steady increase in adsorption