Page 176 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 176
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 172
172 3. Heterogeneous Processes and Reactor Analysis
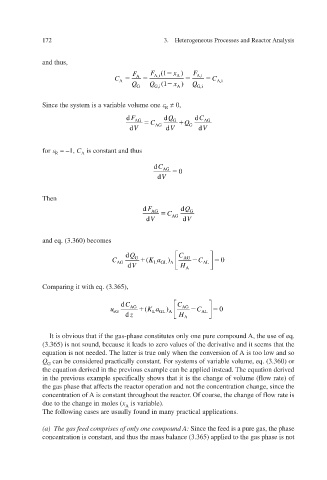
and thus,
F A F A,i (1 x ) F A,i
A
C A C A,i
Q G Q G,i (1 A Q G,i
x )
Since the system is a volume one ariable v ≠ 0,
R
d F d Q d C
AG C G Q AG
d V AG d V G d V
for = –1, C A is constant and thus
R
d C AG 0
d V
Then
d F AG C d Q G
d V AG d V
and eq. (3.360) becomes
Q d C
C G Ka ( ) AG C 0
AG LG A L AL
V d H A
Comparing it with eq. (3.365),
C d C
u AG Ka ( ) AG C 0
L
sG LG A AL
z d H A
It is obvious that if the gas-phase constitutes only one pure compound the use of eq. A,
(3.365) is not sound, because it leads to zero values of the deri and it seems that the e v ati v
equation is not needed. The latter is true only when the conA is too low and so ersion of v
Q G can be considered practically constant. For systems of variable v eq. (3.360) or olume,
the equation derived in the previous example can be applied instead. The equation derived
in the previous example specifically shows that it is the change of volume (flow rate) of
the gas phase that affects the reactor operation and not the concentration change, since the
w rate is concentration of A is constant throughout the reactor. Of course, the change of flo
due to the change in moles ( x A is variable).
The following cases are usually found in many practical applications.
(a) The gas feed comprises of only one compound A: Since the feed is a pure gas, the phase
concentration is constant, and thus the mass balance (3.365) applied to the gas phase is not