Page 174 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 174
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 170
170 3. Heterogeneous Processes and Reactor Analysis
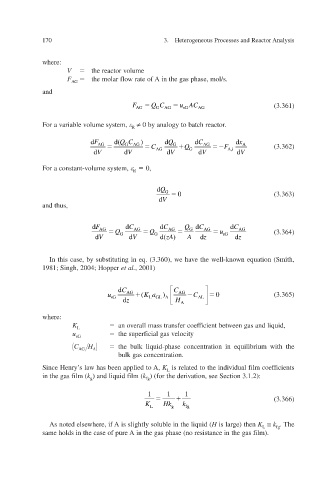
where:
V the reactor v olume
F AG the molar flow rate of A in the gas phase, mol/s.
and
F AG Q C G AG u A AG (3.361)
C
sG
ariable v For a volume system, ≠ 0 by analogy to batch reactor .
R
d F d( QC ) d Q d C d x
AG G AG C G Q AG F A (3.362)
d V d V AG d V G d V A,i d V
For a constant-volume system, 0,
R
d Q G
0 (3.363)
d V
and thus,
d F d C d C Q d C d C
AG Q AG Q AG G AG u AG (3.364)
d V G d V G d( zA ) A d z sG d z
In this case, by substituting in eq. (3.360), we hae the well-known equation (Smith, v
1981; Singh, 2004; Hopper et al. , 2001)
C d AG C AG
u Ka ( ) C 0 (3.365)
L
sG LG A AL
z d H A
where:
K L an oerall mass transfer coeficient between gas and liquid, v f
u sG the superficial gas v elocity
C AG H A the bulk liquid-phase concentration in equilibrium with the
bulk gas concentration.
Since Henry’s law has been applied to A, K L is related to the individual film coef f icients
in the gas film ( k ) and liquid film ( g k ) (for the deri see Section 3.1.2): ation, v
fg
1 1 1
(3.366)
K L Hk g k fg
where, As noted else if A is slightly soluble in the liquid ( H is large) then K L ≅ k . The
fg
same holds in the case of pure A in the gas phase (no resistance in the gas f ilm).