Page 186 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 186
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 182
182 3. Heterogeneous Processes and Reactor Analysis
0.8
0.6
0.4
u (cm/s)
0.2
0
0 10 20 30 40
d (mm) p
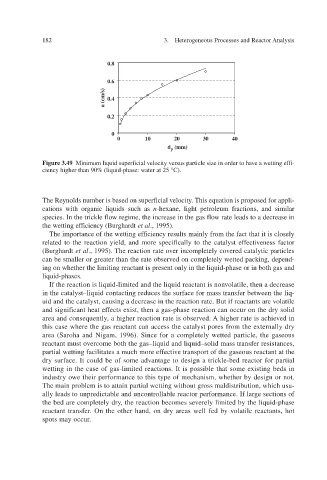
Figure 3.49 Minimum liquid superficial velocity versus particle size in order to have a wetting effi-
ciency higher than 90% (liquid-phase: water at 25 °C).
The Reynolds number is based on superficial velocity. This equation is proposed for appli-
cations with organic liquids such as n -he light petroleum fractions, and similar
xane,
species. In the trickle flow re the increase in the gas flow rate leads to a decrease in
gime,
icienc the wetting efy (Burghardt f et al ., 1995).
The importance of the wetting efy results mainly from the fact that it is closely
f
icienc
related to the reaction yield, and more specifically to the catalyst efeness factor fecti v
v
(Burghardt et al ., 1995). The reaction rate oer incompletely coered catalytic particles v
can be smaller or greater than the rate observed on completely wetted packing, depend-
ing on whether the limiting reactant is present only in the liquid-phase or in both gas and
liquid-phases.
If the reaction is liquid-limited and the liquid reactant is nonolatile, then a decrease
v
in the catalyst–liquid contacting reduces the surface for mass transfer between the liq-
uid and the catalyst, causing a decrease in the reaction rate. But if reactants are volatile
and significant heat effects exist, then a gas-phase reaction can occur on the dry solid
area and consequently a higher reaction rate is observed. A higher rate is achieed in v
,
this case where the gas reactant can access the catalyst pores from the externally dry
area (Saroha and Nigam, 1996). Since for a completely wetted particle, the gaseous
reactant must oercome both the gas–liquid and liquid–solid mass transfer resistances,
v
v
partial wetting facilitates a much more effectie transport of the gaseous reactant at the
dry surface. It could be of some advantage to design a trickle-bed reactor for partial
wetting in the case of gas-limited reactions. It is possible that some existing beds in
industry owe their performance to this type of mechanism, whether by design or not.
The main problem is to attain partial wetting without gross maldistribution, which usu-
ally leads to unpredictable and uncontrollable reactor performance. If large sections of
the bed are completely dry the reaction becomes seerely limited by the liquid-phase
,
v
reactant transfer. On the other hand, on dry areas well fed by volatile reactants, hot
spots may occur .