Page 21 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 21
Else_AIEC-INGLE_Ch001.qxd 7/13/2006 1:53 PM Page 17
1.1 Introduction 17
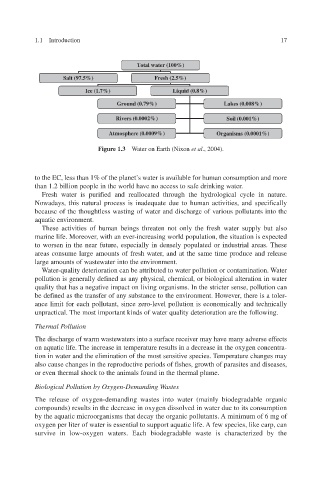
Total water (100%)
Salt (97.5%) Fresh (2.5%)
Ice (1.7%) Liquid (0.8%)
Ground (0.79%) Lakes (0.008%)
Rivers (0.0002%) Soil (0.001%)
Atmosphere (0.0009%) Organisms (0.0001%)
Figure 1.3 Water on Earth (Nixon et al ., 2004).
ater is a to the EC, less than 1% of the planet’ s wvailable for human consumption and more
than 1.2 billion people in the world hae no access to safe drinking w v . ater
Fresh water is purified and reallocated through the hydrological cycle in nature.
Nowadays, this natural process is inadequate due to human acti and specif ically
vities,
because of the thoughtless wasting of water and discharge of various pollutants into the
aquatic environment.
These activities of human beings threaten not only the fresh water supply but also
er v marine life. Moreo, with an e the situation is e er -increasing w orld population, v xpected
to worsen in the near future, especially in densely populated or industrial areas. These
areas consume large amounts of fresh w and at the same time produce and release
ater
,
large amounts of water into the en w vironment. aste
Water-quality deterioration can be attributed to water pollution or contamination. Water
pollution is generally defined as any physical, chemical, or biological alteration in w ater
quality that has a nee impact on living organisms. In the stricter sense, pollution can
gati
v
,
be defined as the transfer of any substance to the environment. Ho there is a toler- v we er
v
ance limit for each pollutant, since zero-leel pollution is economically and technically
unpractical. The most important kinds of water quality deterioration are the follo wing.
Thermal Pollution
The discharge of warm wastewaters into a surface receiver may have many adverse effects
on aquatic life. The increase in temperature results in a decrease in the oxygen concentra-
tion in water and the elimination of the most sensitiT v emperature changes may e species.
also cause changes in the reproductie periods of f growth of parasites and diseases, v ishes,
or even thermal shock to the animals found in the thermal plume.
Biological Pollution by Oxygen-Demanding Wastes
The release of oxygen-demanding wastes into water (mainly biodegradable organic
compounds) results in the decrease in oxygen dissolved in water due to its consumption
by the aquatic microorganisms that decay the organic pollutants. A minimum of 6 mg of
oxygen per liter of water is essential to support aquatic life. w species, like carp, can
A fe
v
survie in low-oxygen waters. Each biodegradable waste is characterized by the