Page 28 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 28
Else_AIEC-INGLE_Ch001.qxd 7/13/2006 1:53 PM Page 24
24 1. Air and ater Pollution W
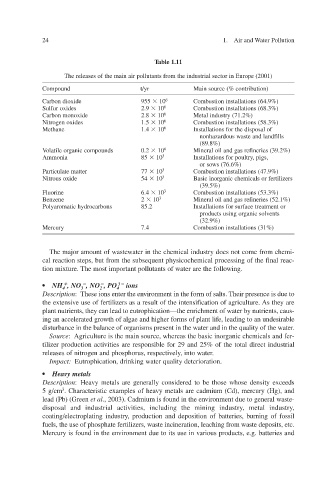
Table 1.11
The releases of the main air pollutants from the industrial sector in Europe (2001)
Compound t/yr Main source (% contrib ution)
Carbon dioxide 955 10 6 Combustion installations (64.9%)
Sulfur oxides 2.9 10 6 Combustion installations (68.3%)
Carbon monoxide 2.8 10 6 Metal industry (71.2%)
Nitrogen oxides 1.5 10 6 Combustion installations (58.3%)
Methane 1.4 10 6 Installations for the disposal of
nonhazardous waste and landf ills
(89.8%)
Volatile organic compounds 0.2 10 6 Mineral oil and gas refineries (39.2%)
Ammonia 85 10 3 Installations for poultry, pigs,
or sows (76.6%)
Particulate matter 77 10 3 Combustion installations (47.9%)
Nitrous oxide 54 10 3 Basic inorganic chemicals or fertilizers
(39.5%)
Fluorine 6.4 10 3 Combustion installations (53.3%)
Benzene 2 10 3 Mineral oil and gas refineries (52.1%)
Polyaromatic hydrocarbons 85.2 Installations for surface treatment or
products using organic solv ents
(32.9%)
Mercury 7.4 Combustion installations (31%)
The major amount of water in the chemical industry does not come from chemi- aste
w
cal reaction steps, but from the subsequent physicochemical processing of the final reac-
tion mixture. The most important pollutants of water are the follo wing.
• H N ,N O ,N O ,P O 3 ions
4 3 2 4
Description : These ions enter the en Their presence is due to vironment in the form of salts.
xtensi the eve use of fertilizers as a result of the intensification of agriculture. As they are
y can lead to eutrophication—the enrichment of water by nutrients, plant nutrients, the caus-
ing an accelerated growth of algae and higher forms of plant life, leading to an undesirable
disturbance in the balance of organisms present in the water and in the quality of the water.
Source : Agriculture is the main source, whereas the basic inorganic chemicals and fer-
tilizer production activities are responsible for 29 and 25% of the total direct industrial
releases of nitrogen and phosphorus, respecti into w , ely v ater .
Impact: Eutrophication, drinking water quality deterioration.
• Heavy metals
Description : Heavy metals are generally considered to be those whose density e xceeds
5 g/cm 3 . Characteristic examples of heavy metals are cadmium (Cd), mercury (Hg), and
lead (Pb) (Green et al aste- ., 2003). Cadmium is found in the en vironment due to general w
,
disposal and industrial activities, including the mining industry metal industry ,
coating/electroplating industry, production and deposition of batteries, burning of fossil
fuels, the use of phosphate fertilizers, wleaching from wetc. aste incineration, aste deposits,
Mercury is found in the environment due to its use in v e.g. batteries and arious products,