Page 322 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 322
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 318
318 4. Adsorption and Ion Exchange
4
3.5
3
2.5
2
1.5
1
0.5 IA
IB
0
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
Fr
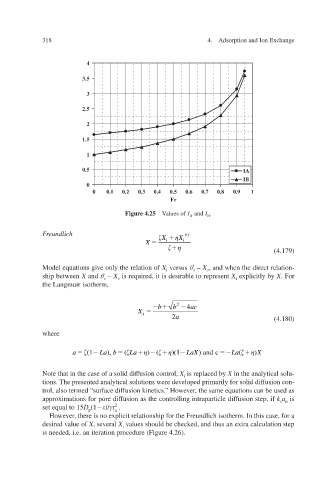
Figure 4.25 Values of I A and I . B
Freundlich X X Fr
X i i
(4.179)
v Model equations gie only the relation of X versus – X , and when the direct relation-
i
ship between X and – X is required, it is desirable to represent X i explicitly by X . F or
the Langmuir isotherm,
b b 2 ac 4
X i
a 2 (4.180)
where
a (1 La b ), ( La ) )(1 X La ) a c nd La( ) X
(
Note that in the case of a solid dif fusion control, X is replaced by X in the analytical solu-
i
tions. The presented analytical solutions were developed primarily for solid diffusion con-
trol, also termed “surface dif Ho the same equations can be used as , er we v
fusion kinetics.
”
approximations for pore diffusion as the controlling intraparticle diffusion step, if k a is
s u
2
r
)/
set equal to 15 D (1 .
p o
However, there is no e for a xplicit relationship for the Freundlich isotherm. In this case,
desired value of X ,s l e a r v e X ed, values should be check and thus an extra calculation step
i
is needed, i.e. an iteration procedure (Figure 4.26).