Page 57 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 57
Else_AIEC-INGLE_cH002.qxd 6/20/2006 11:32 AM Page 53
Adsorption,
vironmental
2.4 En Ion Exchange, and Catalysis 53
Applications of
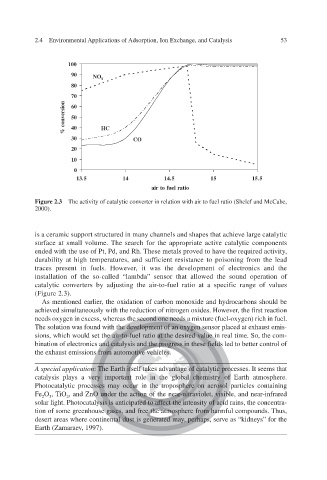
100
90 NO x
80
70
60
50
40 HC
% conversion
30 CO
20
10
0
13.5 14 14.5 15 15.5
air to fuel ratio
Figure 2.3 The activity of catalytic converter in relation with air to fuel ratio (Shelef and McCabe,
2000).
is a ceramic support structured in many channels and shapes that achiee large catalytic v
v
surface at small volume. The search for the appropriate actie catalytic components
ended with the use of Pt, Pd, and Rh. These metals proed to hae the required activity v v ,
f
durability at high temperatures, and suficient resistance to poisoning from the lead
,
v
traces present in fuels. Howeer it was the deelopment of electronics and the
v
installation of the so-called “lambda” sensor that allowed the sound operation of
v
catalytic conerters by adjusting the air-to-fuel ratio at a specific range of values
(Figure 2.3).
,
As mentioned earlier the oxidation of carbon monoxide and hydrocarbons should be
achieved simultaneously with the reduction of nitrogen oxides. However, the f irst reaction
needs oxygen in excess, whereas the second one needs a mixture (fuel-oxygen) rich in fuel.
The solution was found with the development of an oxygen sensor placed at exhaust emis-
sions, which would set the air-to-fuel ratio at the desired value in real time. So, the com-
bination of electronics and catalysis and the progress in these fields led to better control of
ehicles. e v v the exhaust emissions from automoti
A special application : The Earth itself tak es adv antage of catalytic processes. It seems that
catalysis plays a very important role in the global chemistry of Earth atmosphere.
Photocatalytic processes may occur in the troposphere on aerosol particles containing
violet,
-ultra
Fe 2 O 3 ,T O i 2 , and ZnO under the action of the near visible, and near -infrared
solar light. Photocatalysis is anticipated to af the concentra- fect the intensity of acid rains,
tion of some greenhouse gases, and free the atmosphere from harmful compounds. Thus,
desert areas where continental dust is generated may perhaps, serve as “kidne for the ys”
,
Earth (Zamaraev, 1997).